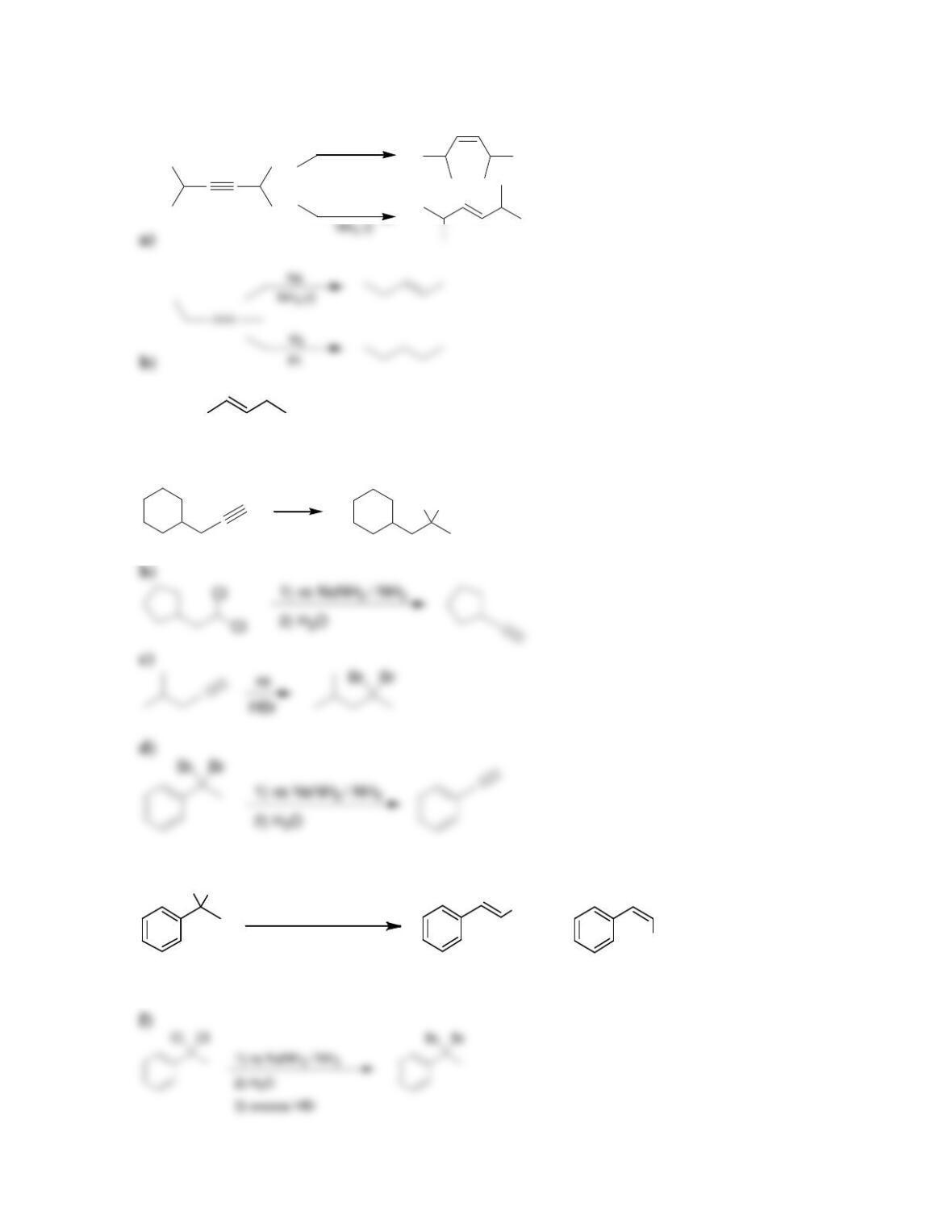
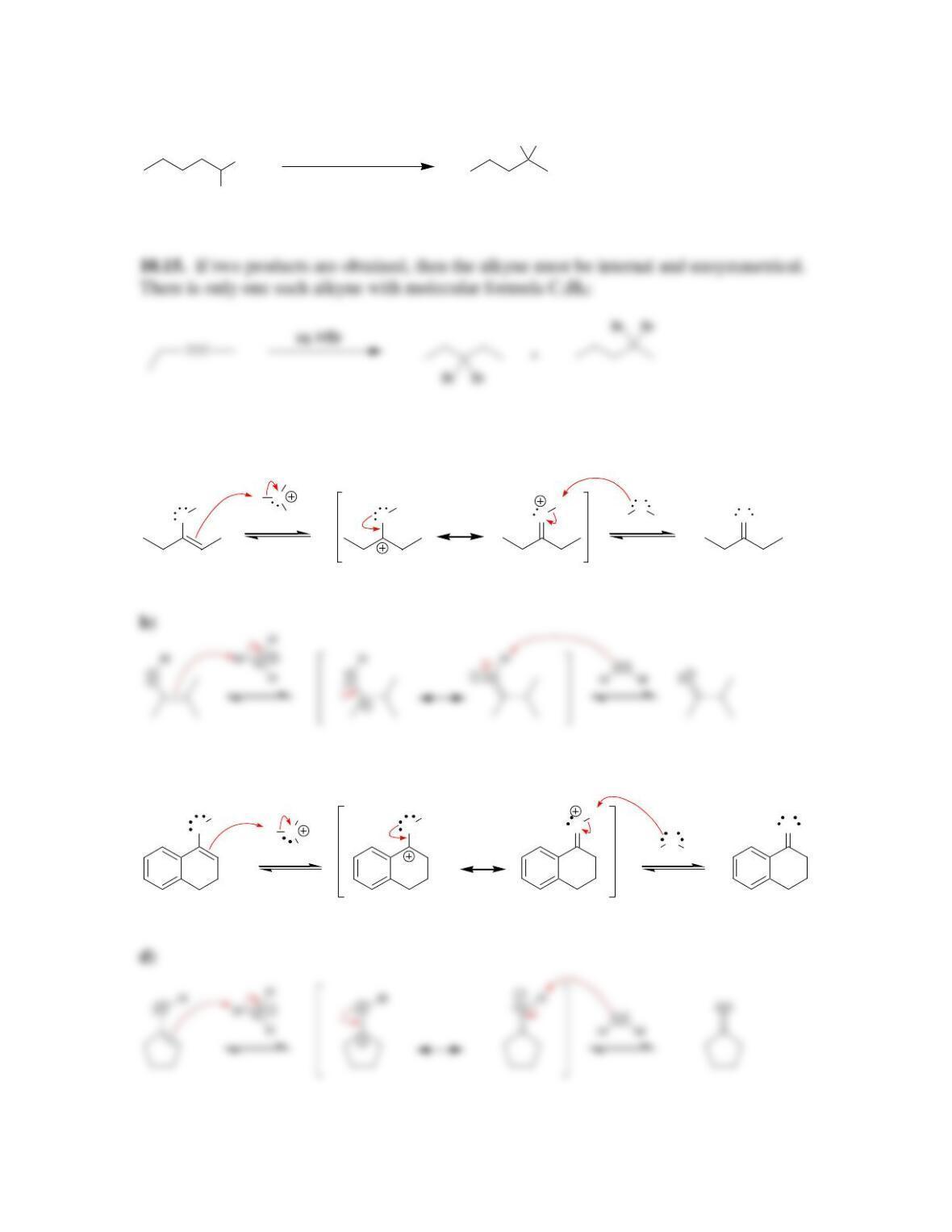
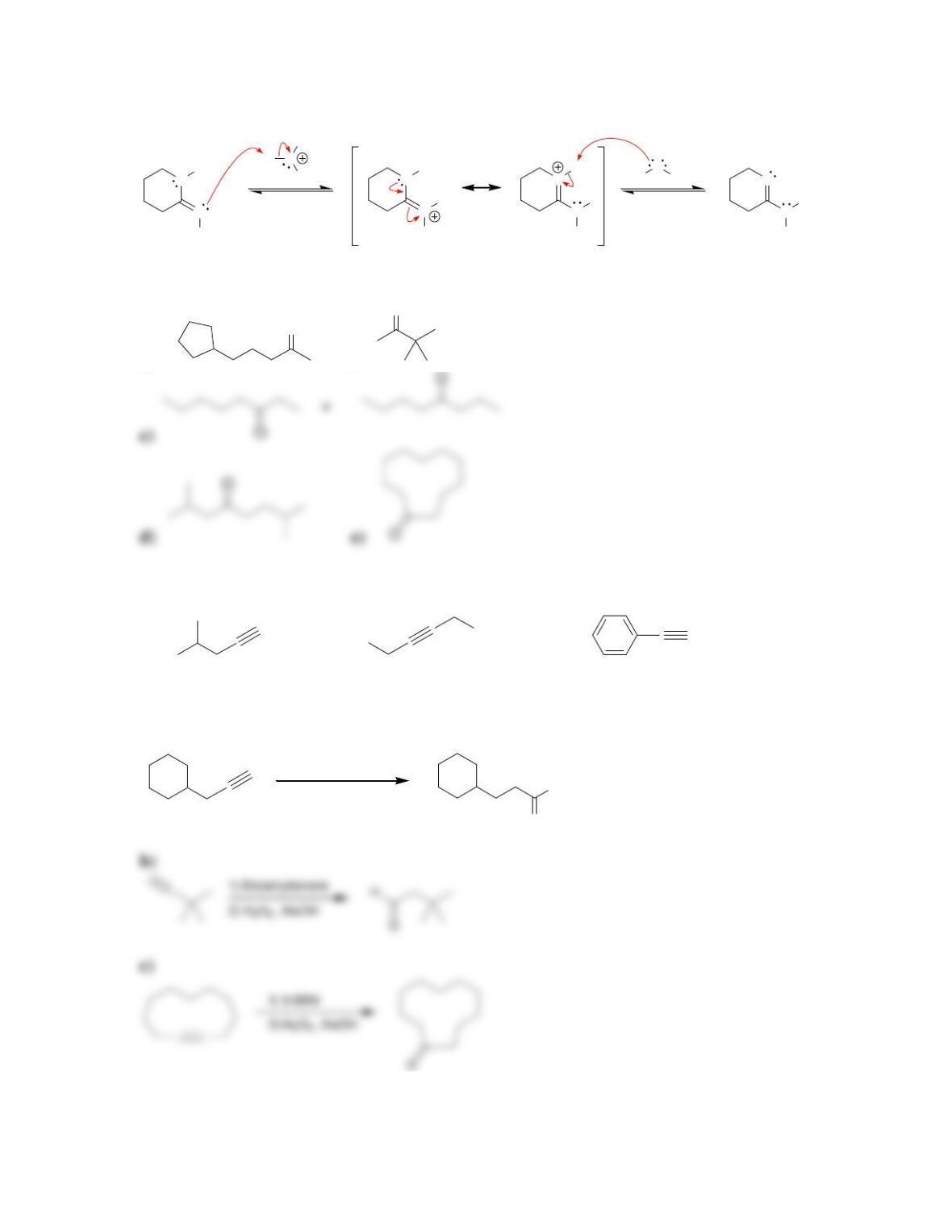
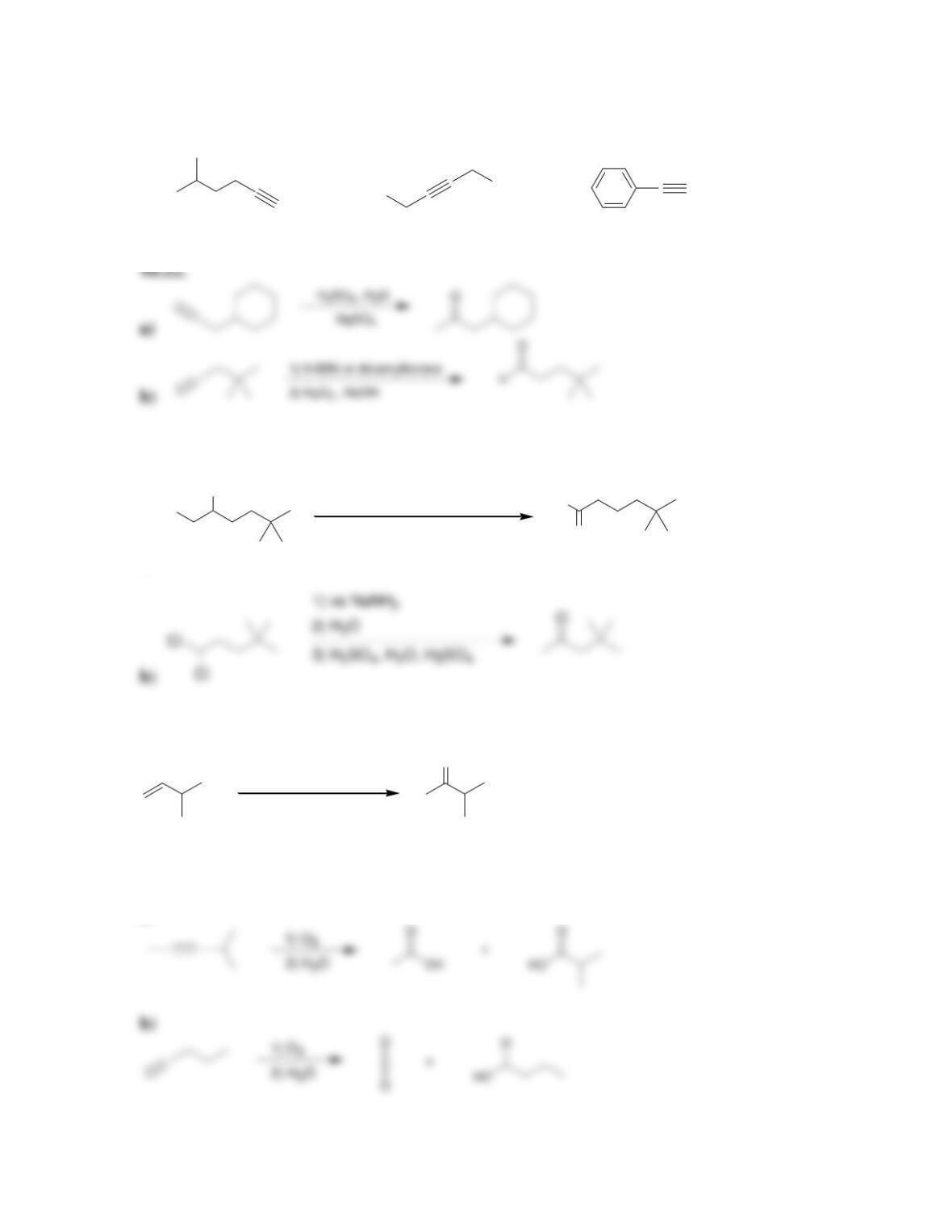
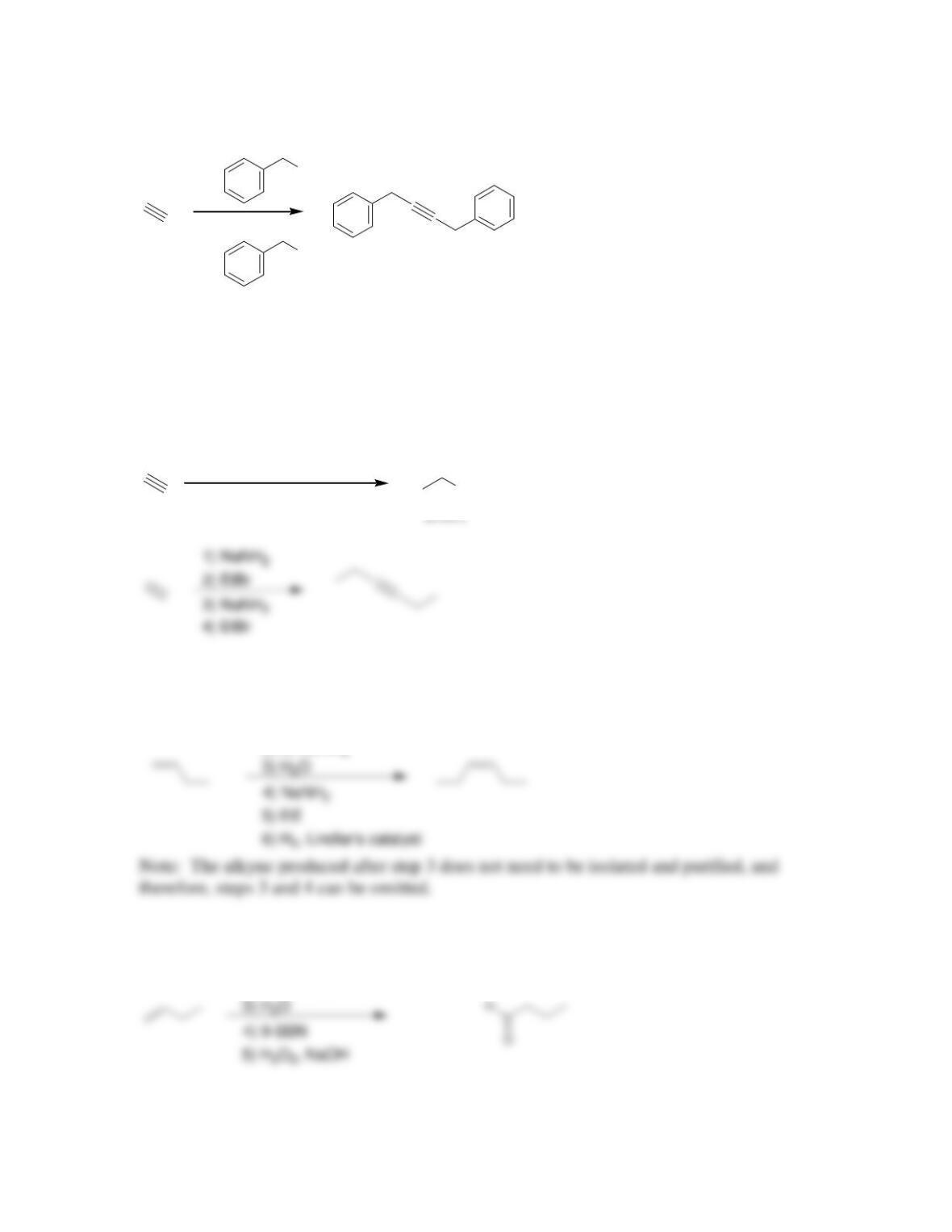
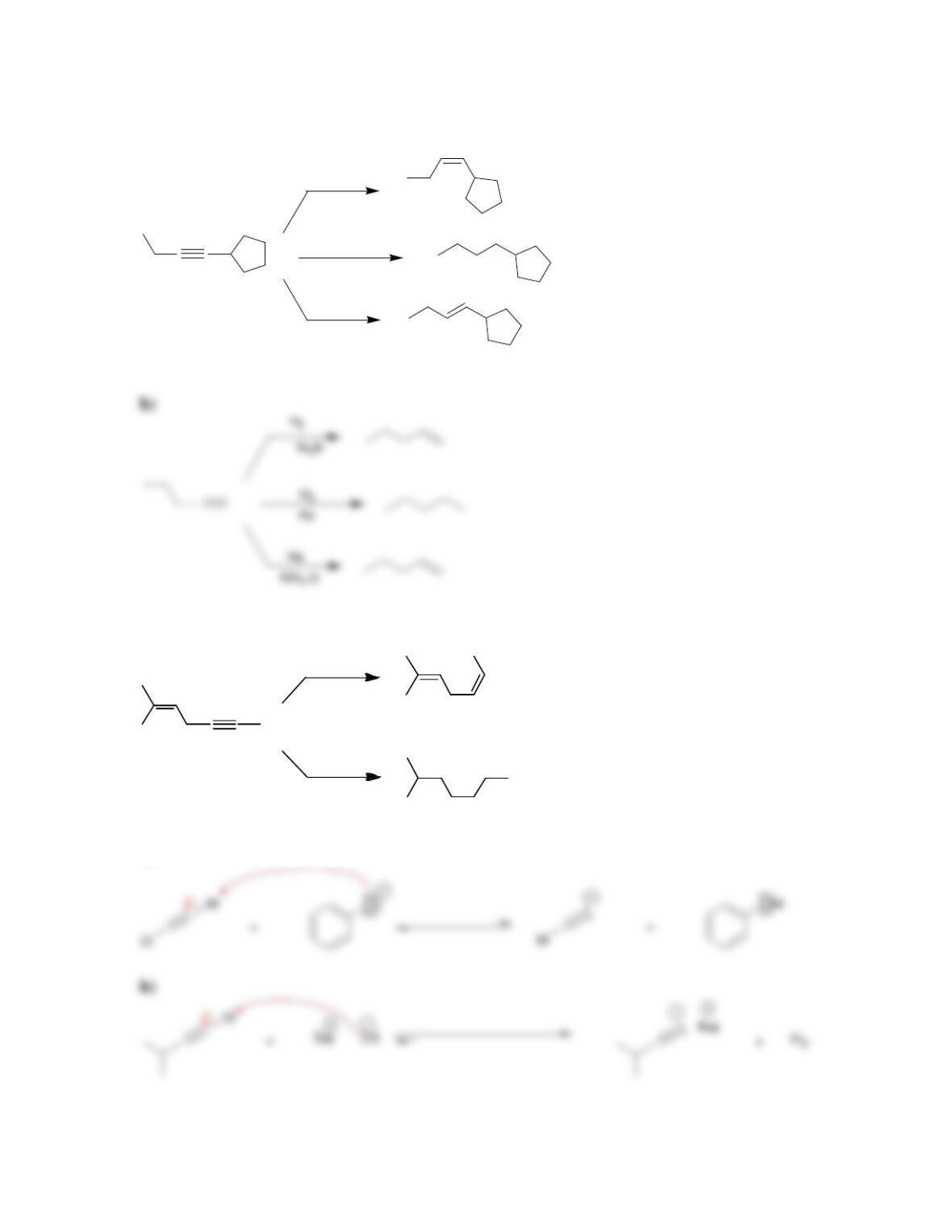
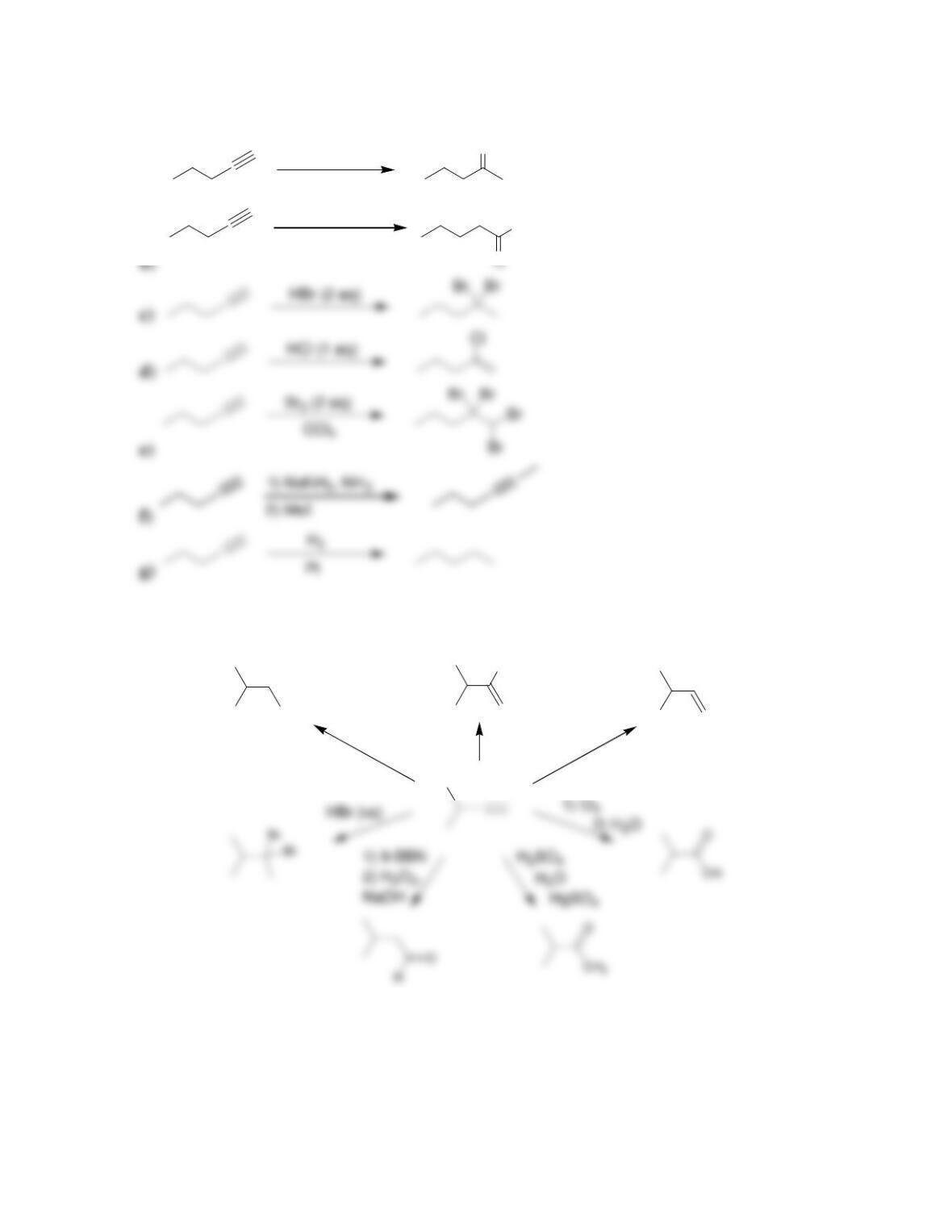
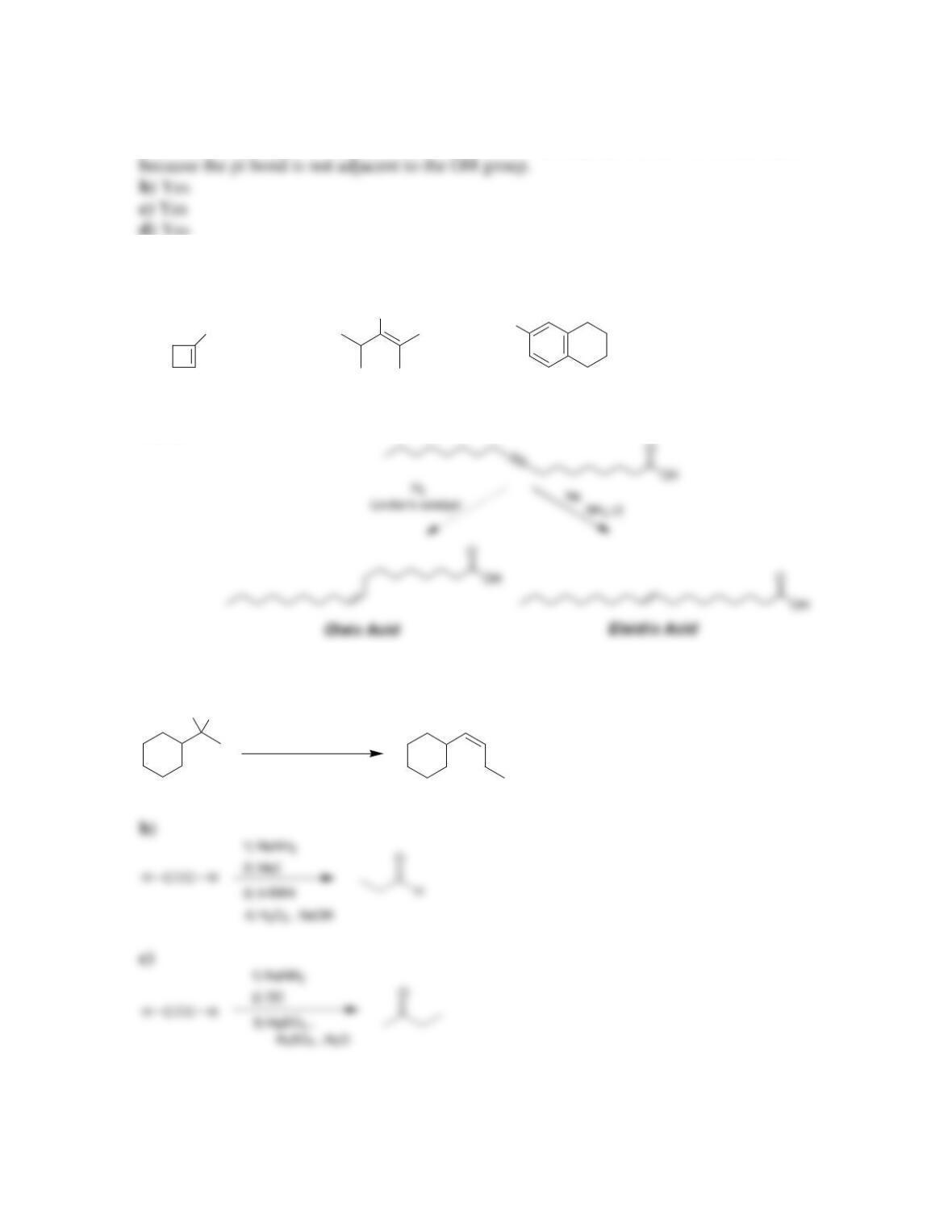
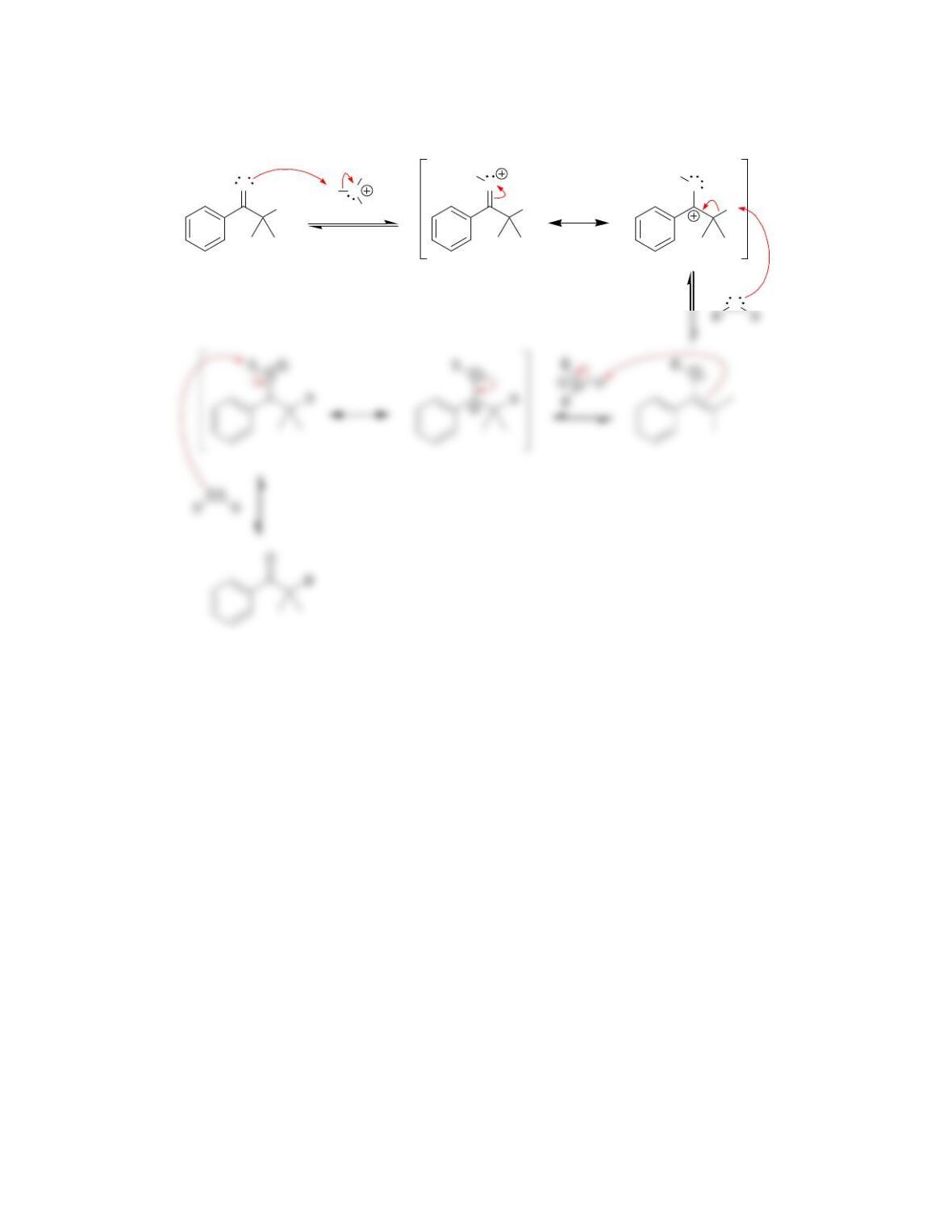
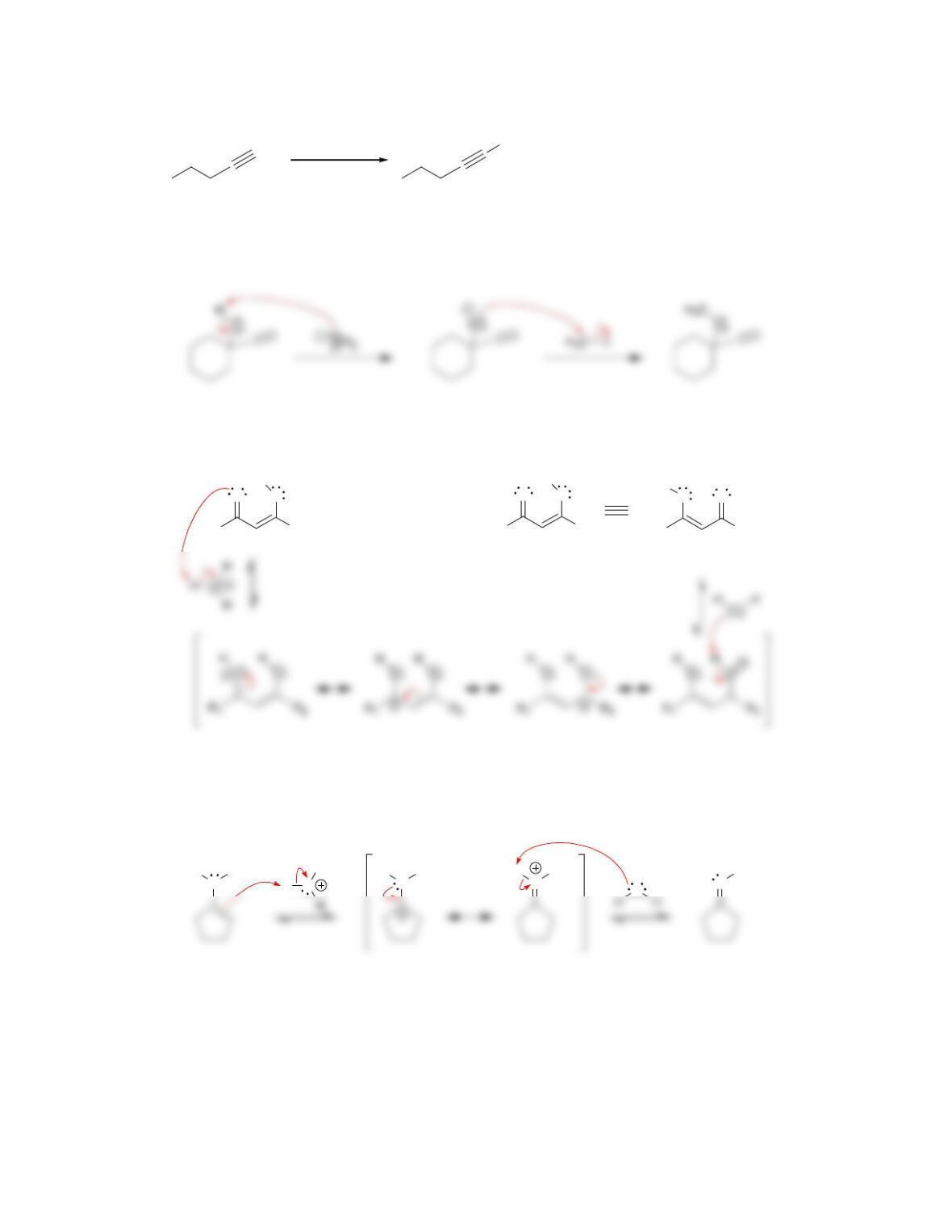Chapter 10
Alkynes
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 10. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• A triple bond is comprised of three separate bonds: one ____ bond and two ____
bonds.
• Alkynes exhibit __________ geometry and can function either as bases or as
___________________.
• Monosubstituted alkynes are terminal alkynes, while disubstituted alkynes are
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 10. The answers appear in the section entitled
SkillBuilder Review.
10.1 Assembling the Systematic Name of an Alkyne
PROVIDE A SYSTEMATIC NAME FOR THE FOLLOWING COMPOUND
1) IDENTIFY THE PARENT
2) IDENTIFY AND NAME SUBSTITUENTS
3) ASSIGN LOCANTS TO EACH SUBSTITUENT
4) ALPHABETIZE