2
CHAPTER 1
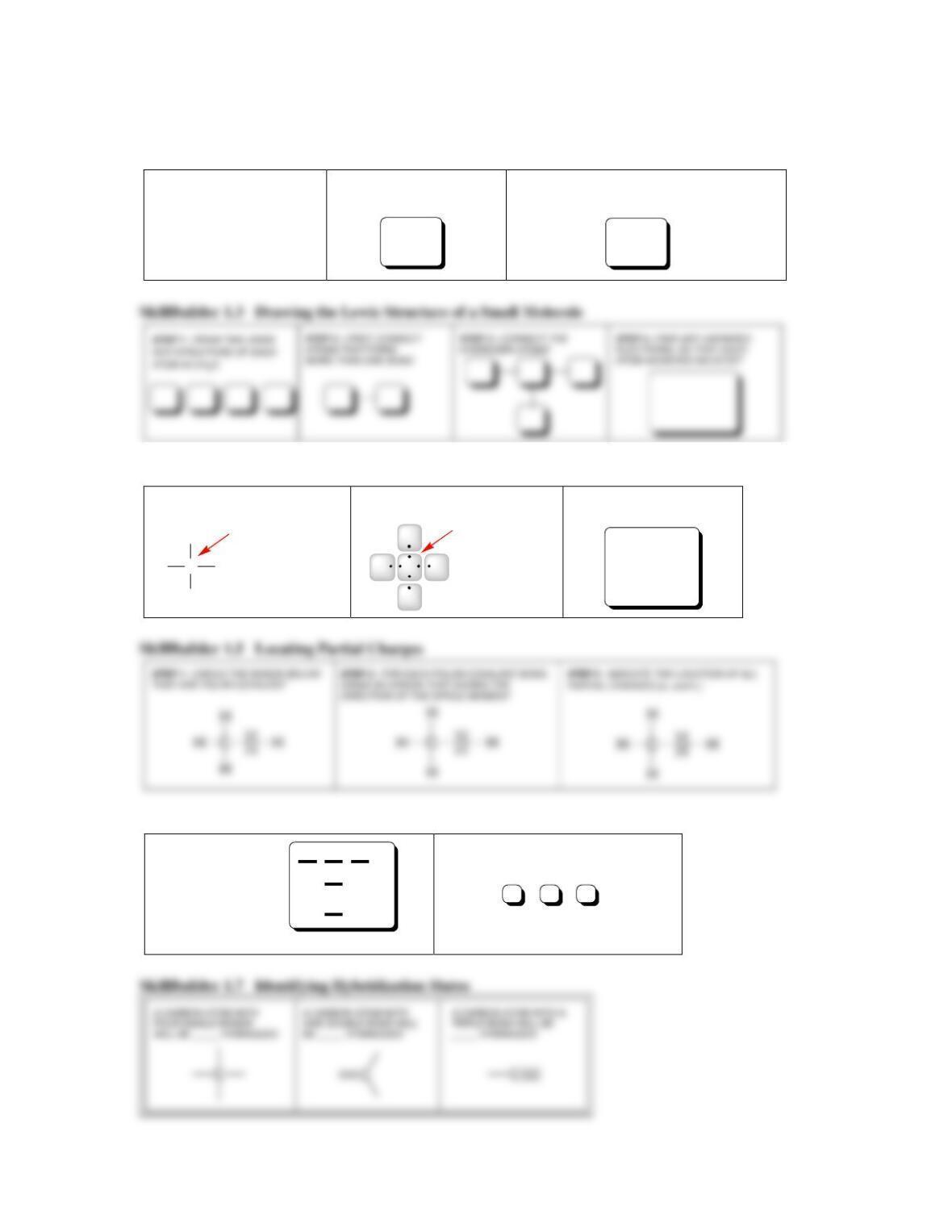
SkillBuilder 1.2 Drawing the Lewis Dot Structure of an Atom
STEP 1 - DETERMINE THE NUMBER
OF VALENCE ELECTRONS
STEP 2 - PLACE ONE ELECTRON
BY ITSELF ON EACH SIDE OF
THE ATOM
STEP 3 - IF THE ATOM HAS MORE THAN FOUR
VALENCE ELECTRONS, PAIR THE REMAINING
ELECTRONS WITH THE ELECTRONS ALREADY DRAWN
Nitrogen is in Group ___ of the
periodic table, and is expected
to have ___ valence electrons.
SkillBuilder 1.4 Calculating Formal Charge
H
H
N
H
H
HN
H
H
H
STEP 1 - DETERMINE THE APPROPRIATE
NUMBER OF VALENCE ELECTRONS
STEP 2 - DETERMINE THE NUMBER OF
VALENCE ELECTRONS IN THIS CASE
STEP 3 - ASSIGN A FORMAL
CHARGE TO THE NITROGEN ATOM
IN THIS CASE
Nitrogen is in
Group ___ of the
periodic table,
and is expected
to have ___
valence electrons.
In this case, the
nitrogen atom is
using only ___
valence electrons.
SkillBuilder 1.6 Identifying Electron Configurations
STEP 1 - IN THE ENERGY
DIAGRAM SHOWN HERE,
DRAW THE ELECTRON
CONFIGURATION OF
NITROGEN (USING
ARROWS TO REPRESENT
ELECTRONS).
STEP 2 - FILL IN THE BOXES BELOW WITH THE
NUMBERS THAT CORRECTLY DESCRIBE THE
ELECTRON CONFIGURATION OF NITROGEN
1s
2s
2p
1s
2s
2p
Nitrogen