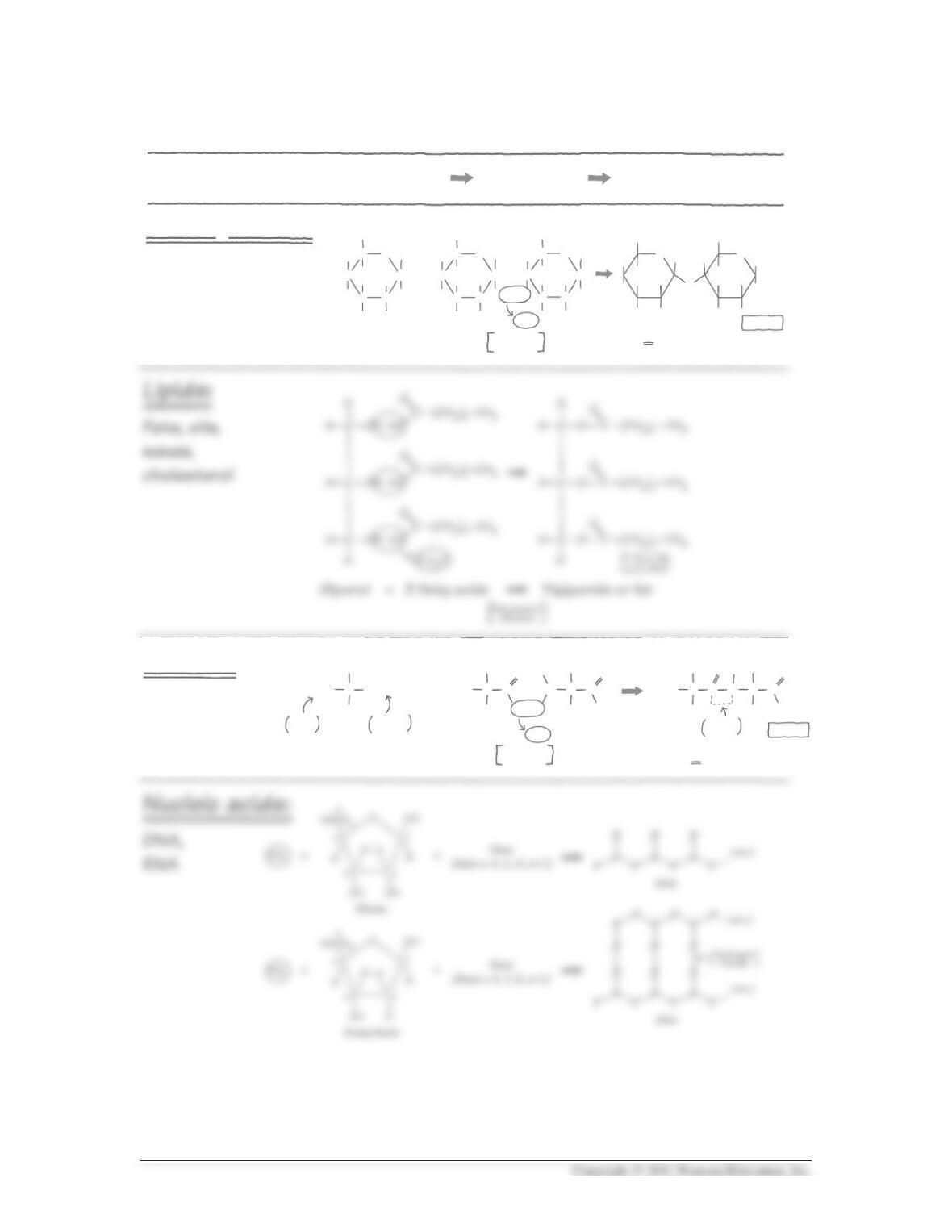
5. Functional groups can modify the properties of organic molecules. In the following
table, indicate whether each functional group is polar or nonpolar and hydrophobic
or hydrophilic. Which of these functional groups are found in proteins and lipids?
Activity 4.1/5.1 15
Functional
group
Polar or
nonpolar
Hydrophobic
or hydrophilic
Found in all
proteins
Found in
many proteins
Found in
many lipids
—OH Polar Hydrophilic No In some R
groups
In fatty acids
as terminal
reactive group
6. You want to use a radioactive tracer that will label only the protein in an RNA virus.
Assume the virus is composed of only a protein coat and an RNA core. Which of the
following would you use? Be sure to explain your answer.
a. Radioactive P b. Radioactive N c. Radioactive S d. Radioactive C
To distinguish between protein and RNA in a virus, you could use radioactively
7. Closely related macromolecules often have many characteristics in common. For
example, they share many of the same chemical elements and functional groups.
Therefore, to separate or distinguish closely related macromolecules, you need to
determine how they differ and then target or label that difference.
a. What makes RNA different from DNA?
RNA contains ribose sugar, whereas DNA contains deoxyribose sugar. In addition,
b. If you wanted to use a radioactive or fluorescent tag to label only the RNA in a cell
and not the DNA, what compound(s) could you label that is/are specific for RNA?