Chapter 21 Simultaneous heat and mass transfer page 21-7
0
2000
0 20406080
T (C)
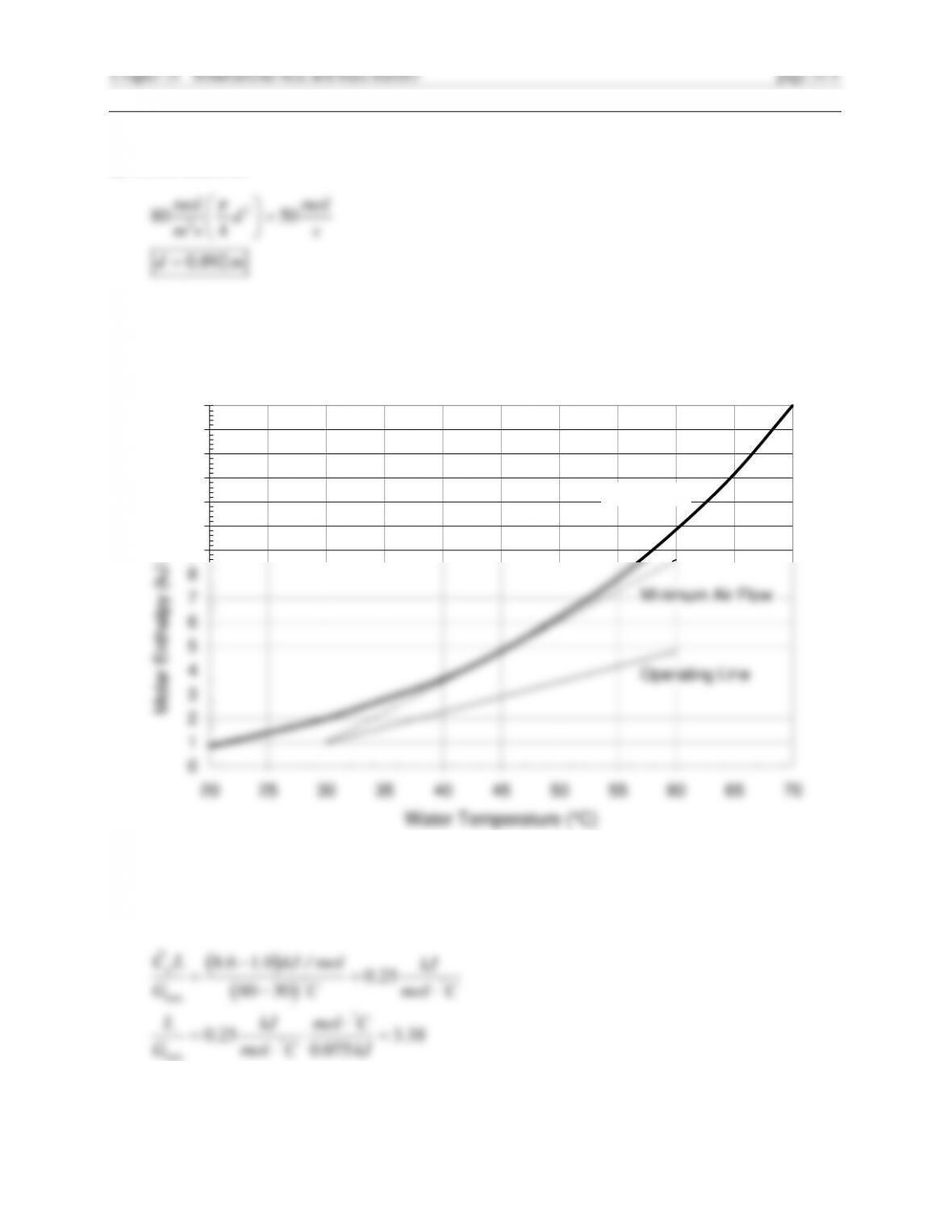
The slope of the operating line is from Eq. 20.3-14. For the water stream,
2
Hence at the top of the tower,
Hin = 702 + 98.7*(66 - 20) = 5243 J/mol
The numerical integration gives
Hout d
H
Hi -
H = 2.65
By evaluating at the average temperature, 43°C, and 1 atm,
c = 1/82.05/316 = 3.8610-5 mol/cm3 = 38.6 mol/m3
k = 80/10/63/38.6*2.655 = 0.00873 m/s = 0.873 cm/s
(b) In this case, the water flow rate is fixed at 1000 kg/min, and the air flow rate is to be
reduced. To solve this problem, we use a trial-and-error approach to get the solution:
(i) assume a value for nair,
(ii) calculate the slope of the operating line,
Hout d
H
(nair/kc)1 =
(nair/nH2O)1