Chapter 17 Homogeneous chemical reactions page 17-1
Chapter 17 Homogeneous chemical reactions
1. Accelerating antibiotic adsorption
The adsorption on carbon is apparently slow, probably due to slow (heterogeneous) reaction or
slow diffusion. Assume this, then
k
0 = 6.110-4 cm/s
The adsorption on ion exchanger are much faster. If it is still diffusion controlled, then from
Eq. 16.1-17,
Given D = 9.410-7 cm2/s, we have
By trial and error, = 112.9 s-1
If the carbon adsorption is not diffusion controlled, the answer will be much less.
2. H2S absorption by monoethanol amine solution
The solution of this type of mass transfer is discussed in detail in D. R. Olander, AIChE J., 6,
(a) Reversible case:
For a reversible reaction H2S + RNH2
RNH3
++ HS-
Let 1 = H2S, 2 = RNH2, 3 = RNH3
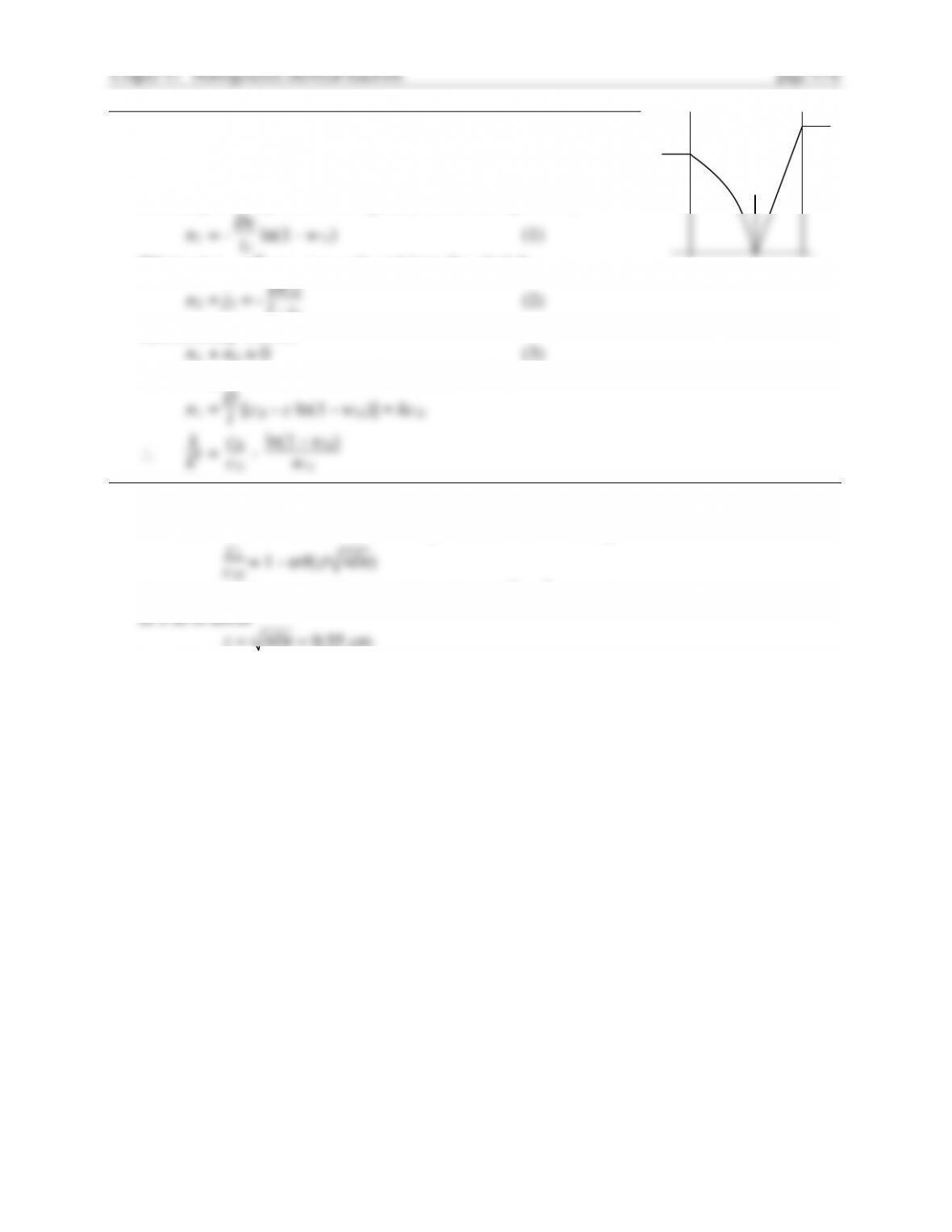
+, 4 = HS We want to derive an equation similar to Eq.
-
where = 1
D4
2
+ 4
D2c2l
D4
Assuming all diffusion coefficients are equal, and c1l = 0, then becomes
2
and Eq. (1) reduces to
k = 1 + K2 + 4Kc2l/c10 - K
2 (2)
Given K = 275, c2l = 0.1 M, H = 545 atm,
Thus
On the other hand, if c2l is in great excess, such that c2l >> Kc10, then Eq. (2) reduces to