Chapter 16 General questions and heterogeneous chemical reactions page 16-4
Mass balance in the ceramic: diffusion flux = reaction rate
9. Interfacial resistance of a molecularly compact interfacial film
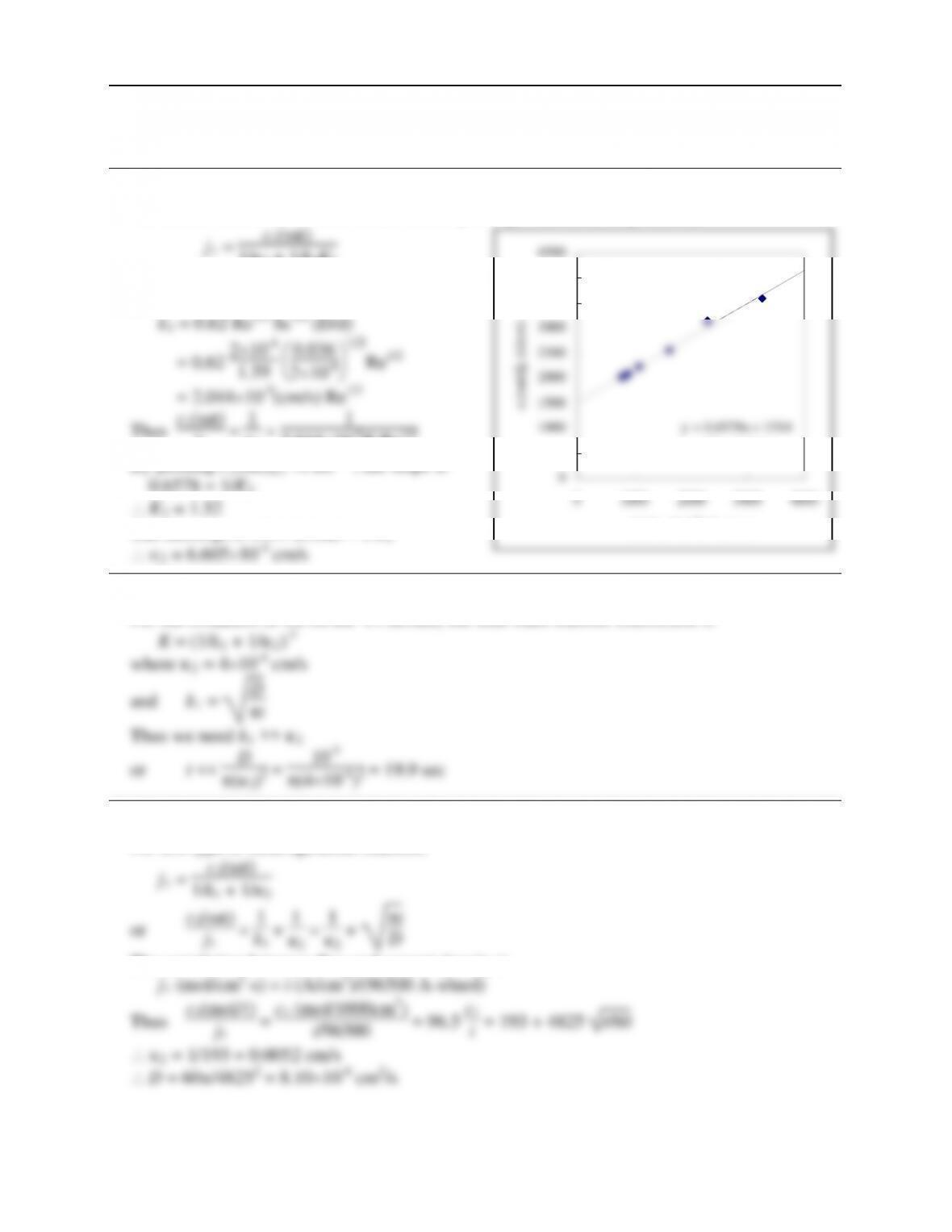
The result is like that for a heterogeneous reaction given in Eq. 16.3-6 and 16.3-7:
k1 + 1
k2 + k-2
k2k3
In the common case, k2 and k-2 are very large, so this reduces to Eq. 8.5-8 to 8.5-12, but with
(k2/k-2) equivalent to the partition coefficient, H. In the exceptional case where k2 is slow, or k-2
is zero, this reduces to the case discussed in Ex. 16.3-2.
10. Mass balance on the hydrate layer
Accumulation rate of the hydrate layer = Diffusion rate of water
11. Air hydrate
For an approximate solution, make a pseudo-steady state mass balance
hydrate accumulation = water diffusion across hydrate
where R and r are the initial and present bubble radii, respectively; k is a mass transfer
coefficient equal to D
R - r; and and are hydrate and water densities, assumed equal.
H H2O