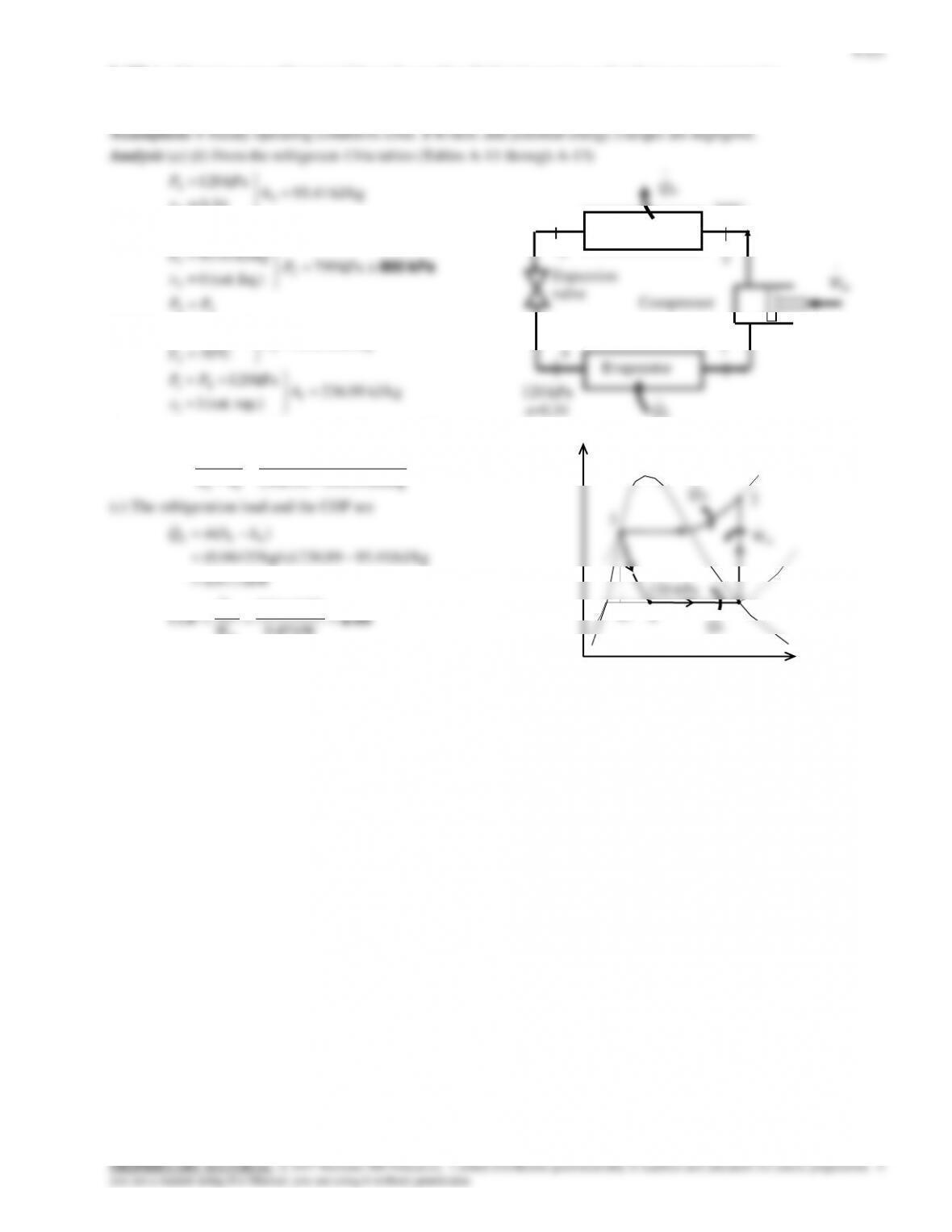
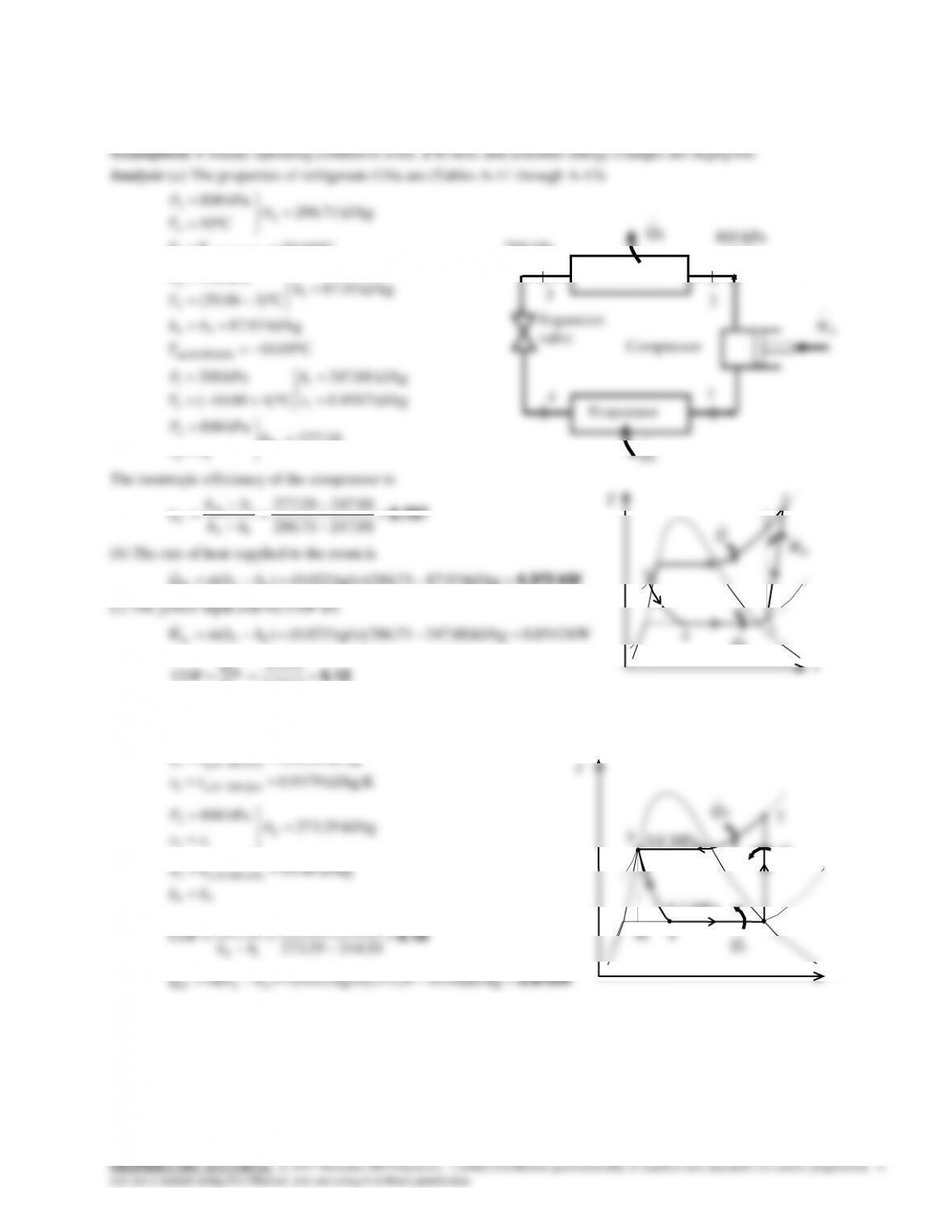
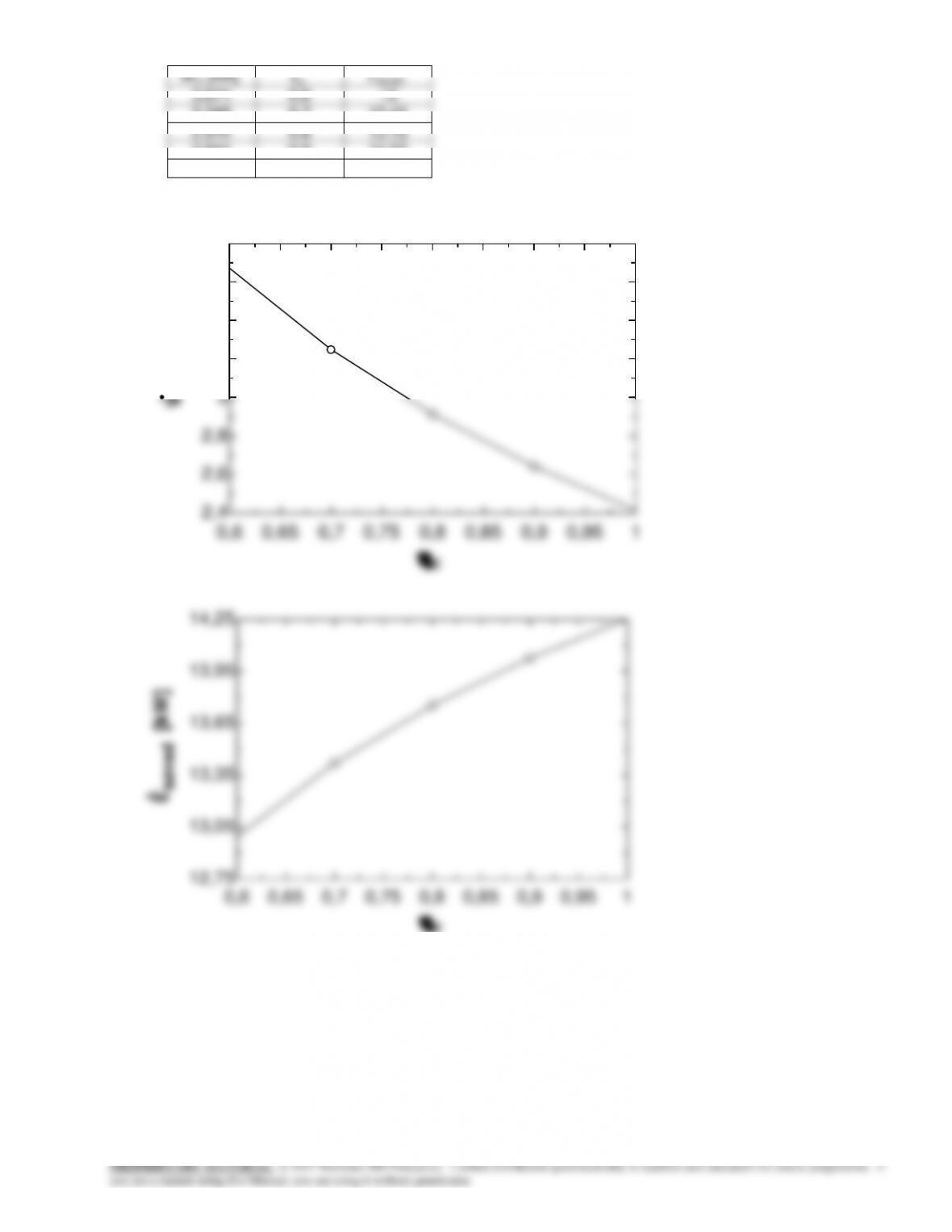
9-163 Problem 9-162 is reconsidered. The effect of the compressor isentropic efficiency on the power input to the
compressor and the electric power saved by using a heat pump rather than electric resistance heating is to be investigated.
Analysis The problem is solved using EES, and the solution is given below.
"Input Data"
"Input Data is supplied in the diagram window"
s[1]=entropy(Fluid$,P=P[1],T=T[1])
h2s=enthalpy(Fluid$,P=P[2],s=s[1]) "Identifies state 2s as isentropic"
h[1]+Wcs=h2s "energy balance on isentropic compressor"
Wc=Wcs/Eta_c"definition of compressor isentropic efficiency"
h[1]+Wc=h[2] "energy balance on real compressor-assumed adiabatic"
"Throttle Valve"
h[4]=h[3] "energy balance on throttle - isenthalpic"
x[4]=quality(Fluid$,h=h[4],P=P[4]) "properties for state 4"
s[4]=entropy(Fluid$,h=h[4],P=P[4])
T[4]=temperature(Fluid$,h=h[4],P=P[4])