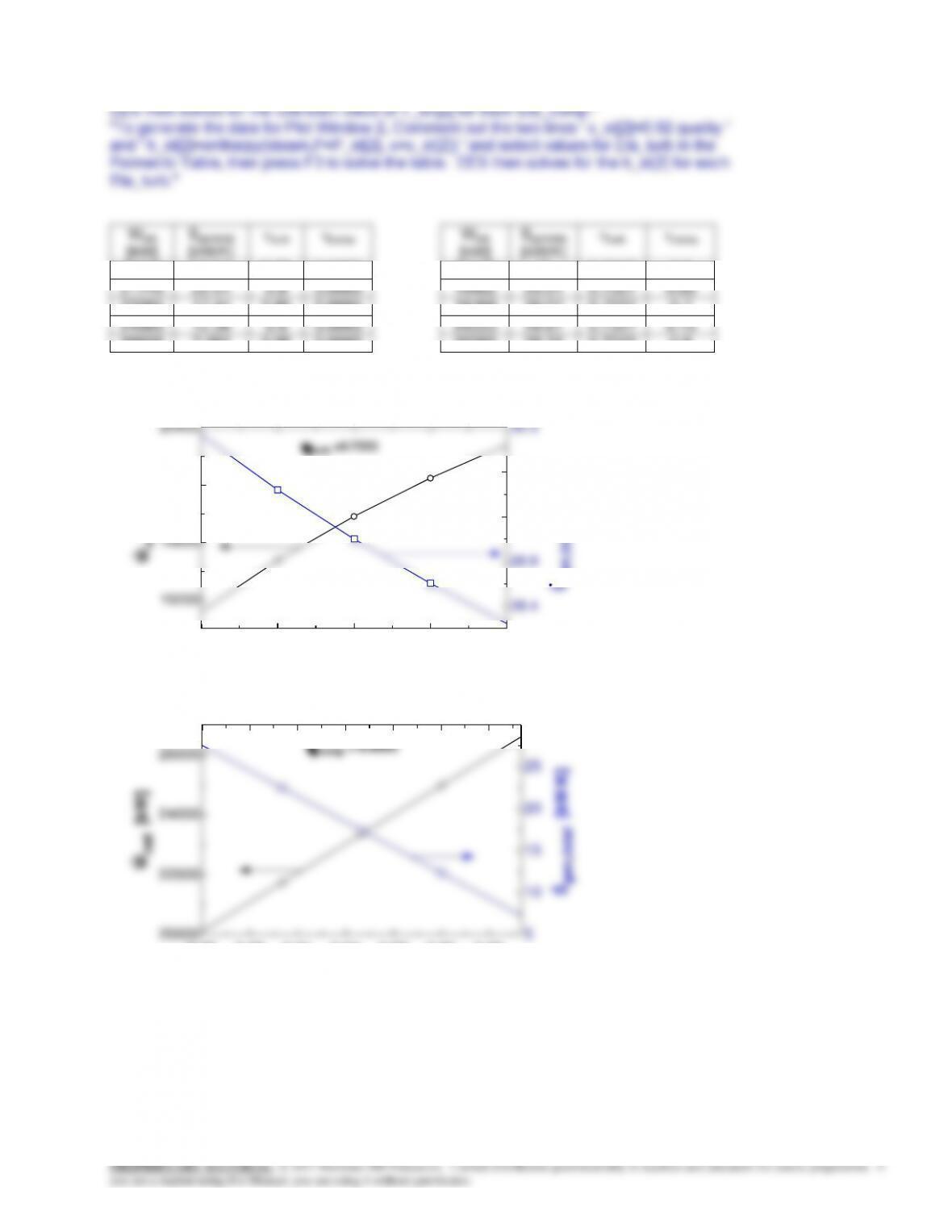
8-179 Problem 8-178 is reconsidered. The isentropic efficiencies for the compressor and turbine are to be determined,
and then the effect of varying the compressor efficiency over the range 0.6 to 0.8 and the turbine efficiency over the range
0.7 to 0.95 on the net work for the cycle and the entropy generated for the process is to be investigated. The net work is to
be plotted as a function of the compressor efficiency for turbine efficiencies of 0.7, 0.8, and 0.9.
Analysis The problem is solved using EES, and the results are tabulated and plotted below.
"Input Data"
m_dot_air = 10 [kg/s] "air compressor (air) data"
"Conservation of mass for the compressor m_dot_air_in = m_dot_air_out =m_dot_air"
"Conservation of energy for the compressor is:"
E_dot_comp_in - E_dot_comp_out = DELTAE_dot_comp
DELTAE_dot_comp = 0 "Steady flow requirement"
E_dot_comp_in=m_dot_air*(enthalpy(air,T=T_air[1])) + W_dot_comp_in
"Conservation of energy for the turbine is:"
E_dot_turb_in - E_dot_turb_out = DELTAE_dot_turb
DELTAE_dot_turb = 0 "Steady flow requirement"
E_dot_turb_in=m_dot_st*h_st[1]
h_st[1]=enthalpy(steam,T=T_st[1], P=P_st[1])
calculated at P_st[2], h_st[2] and not P_st[2] and x_st[2]"
s_st[2]=entropy(steam,P=P_st[2],h=h_st[2])
T_st[2]=temperature(steam,P=P_st[2], h=h_st[2])
s_st_isen[2]=s_st[1]
"Net work done by the process:"