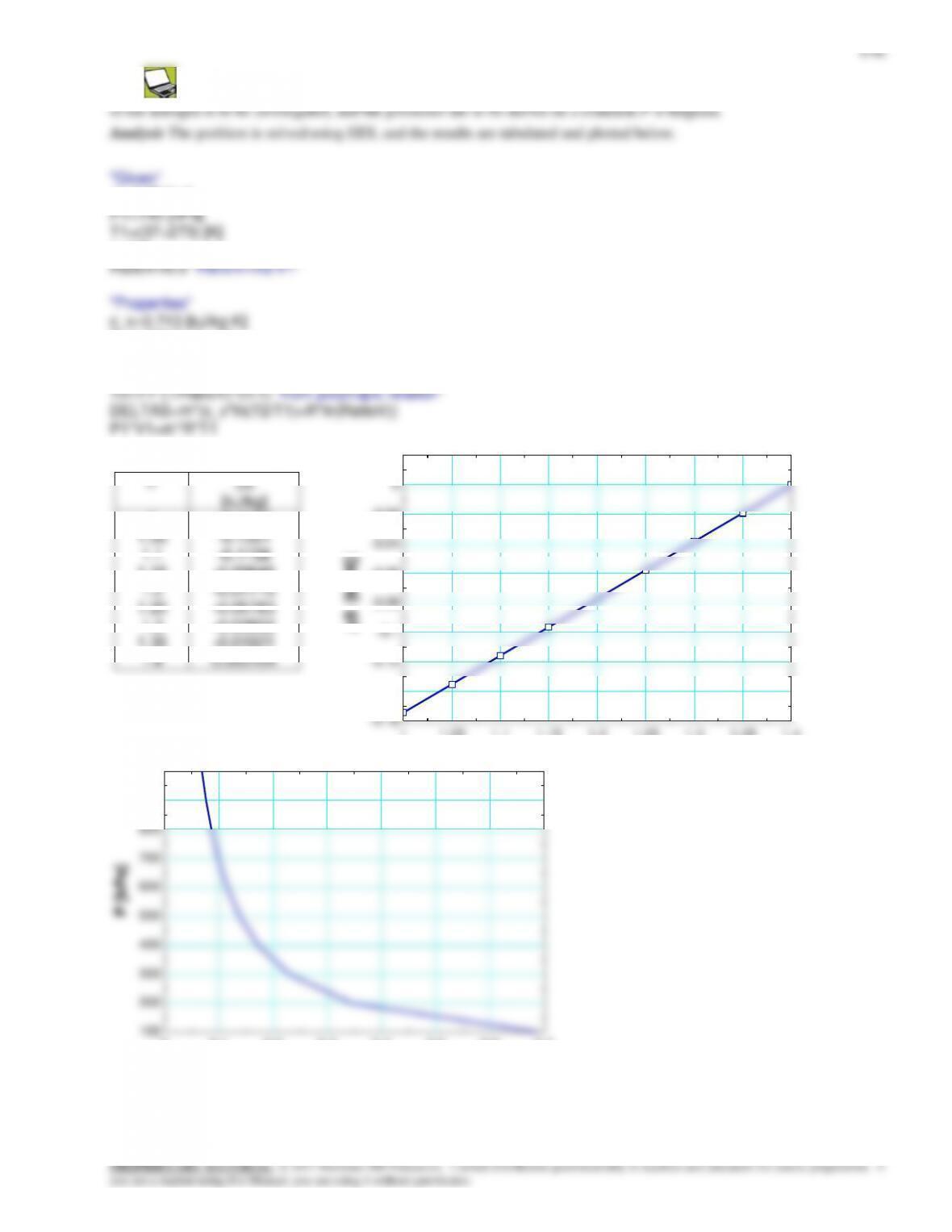
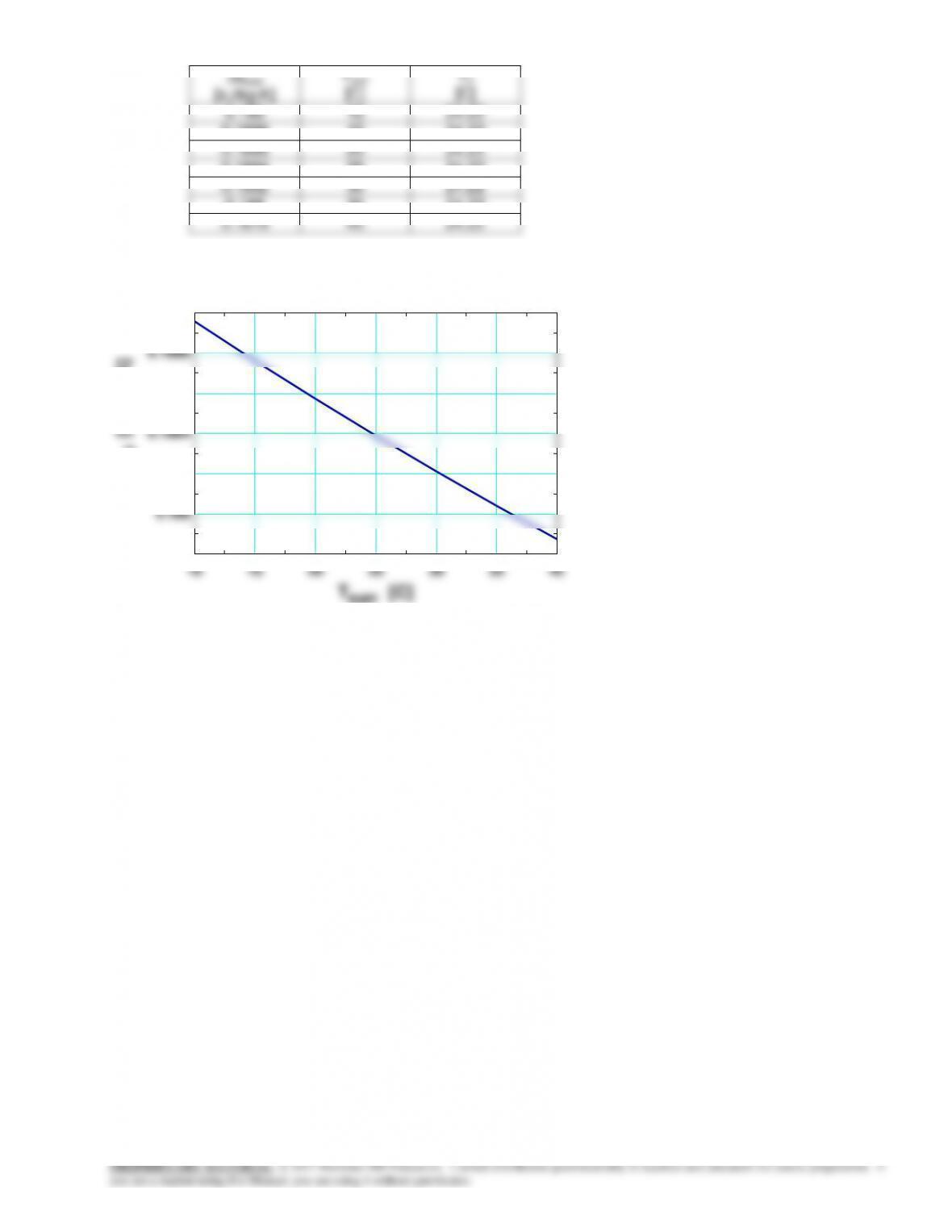
8-82 Problem 8-81 is reconsidered. The effect of varying the surrounding medium temperature from 10°C to 40°C on
the exit temperature and the total entropy change for this process is to be studied, and the results are to be plotted.
Analysis The problem is solved using EES, and the results are tabulated and plotted below.
Function HCal(WorkFluid$, Tx, Px)
"Function to calculate the enthalpy of an ideal gas or real gas"
WorkFluid$ = 'Air'
T[1] = 77 [C]
P[1] = 280 [kPa]
Vel[1] = 50 [m/s]
P[2] = 85 [kPa]
"If we knew the inlet or exit area, we could calculate the mass flow rate. Since we don't know these areas, we
write the conservation of energy per unit mass."
"Conservation of mass: m_dot[1]= m_dot[2]"
"Conservation of Energy - SSSF energy balance for neglecting the change in potential energy, no work, but heat
transfer out is:"