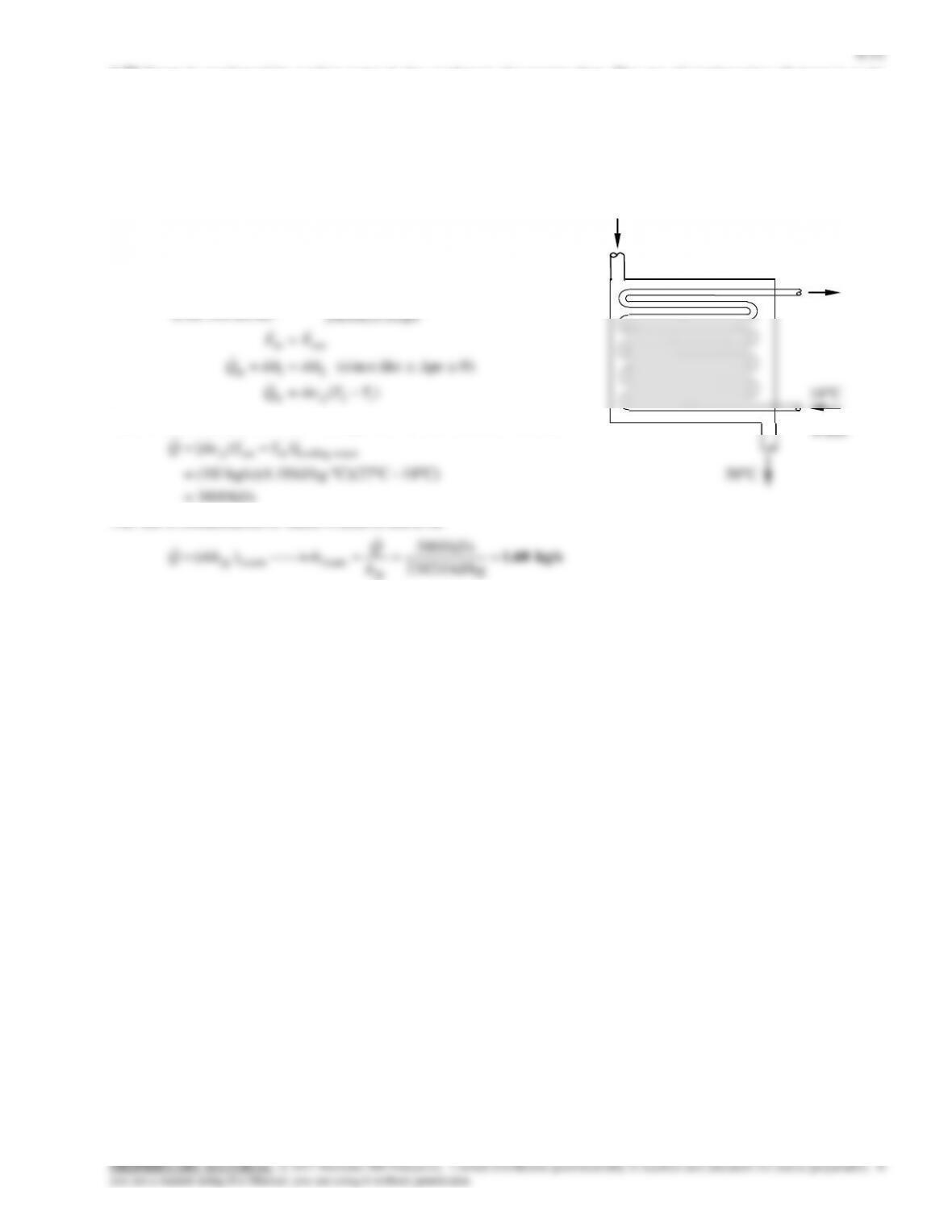
6-71 Oil is to be cooled by water in a thin-walled heat exchanger. The rate of heat transfer in the heat exchanger and the exit
temperature of water is to be determined.
Assumptions 1 Steady operating conditions exist. 2 The heat exchanger is well-insulated so that heat loss to the
surroundings is negligible and thus heat transfer from the hot fluid is equal to the heat transfer to the cold fluid. 3 Changes
in the kinetic and potential energies of fluid streams are negligible. 4 Fluid properties are constant.
Properties The specific heats of water and oil are given to be 4.18
and 2.20 kJ/kg.C, respectively.
Analysis We take the oil tubes as the system, which is a control
volume. The energy balance for this steady-flow system can be
expressed in the rate form as
)(
0)peke (since
0
21out
2out1
outin
energies etc. potential,
kinetic, internal,in change of Rate
(steady) 0
sy stem
mass and work,heat,by
nsferenergy tranet of Rate
outin
TTcmQ
hmQhm
EE
EEE
p
Then the rate of heat transfer from the oil becomes
=C)40CC)(150kJ/kg. kg/s)(2.2 2()]([ oiloutin kW 484 TTcmQ p
Noting that the heat lost by the oil is gained by the water, the outlet temperature of the water is determined from
C99.2
=
C)kJ/kg. kg/s)(4.18 (1.5
kJ/s 484
C22 )]([
water
inoutwaterinout
p
pcm
Q
TTTTcmQ