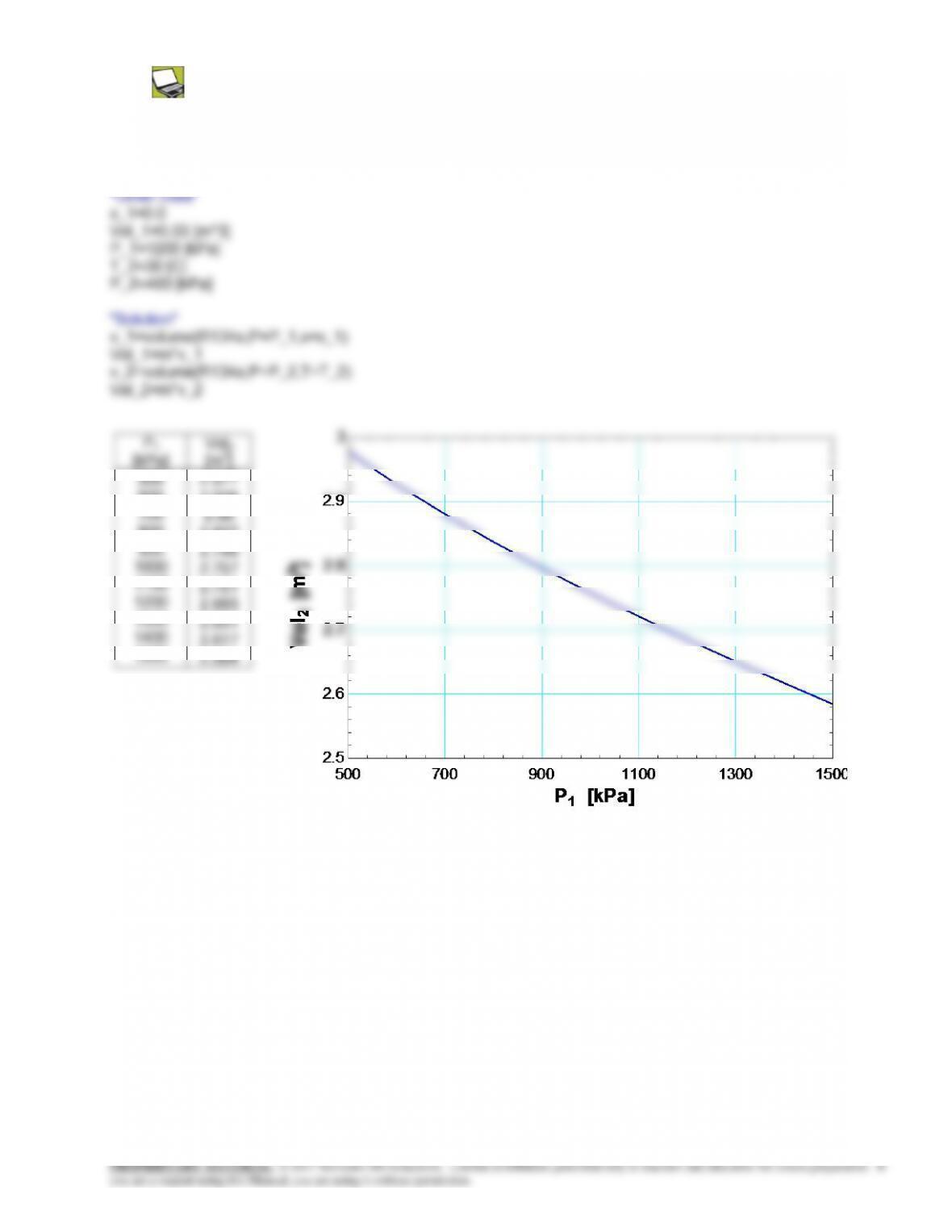
4-106 The temperature of steam in a tank at a specified state is to be determined using the ideal gas relation, the
generalized chart, and the steam tables.
Properties The gas constant, the critical pressure, and the critical temperature of water are, from Table A-1,
MPa 22.06 K, 647.1 ,K/kgmkPa 0.4615
crcr
3
==⋅⋅= PTR
Analysis (a) From the ideal gas equation of state,
⋅⋅
K) K)(673/kgmkPa (0.4615
3
RT
57.0
1.48
K) K)(647.1/kgmkPa (0.4615
kPa) 0/kg)(22,06m (0.02
/
040.1
K 647.1
K 673
3
3
crcr
actual
cr
=
=
⋅⋅
==
===
R
R
R
P
PRT
T
T
T
v
v
4-107 One section of a tank is filled with saturated liquid R-134a while the other side is evacuated. The partition is
removed, and the temperature and pressure in the tank are measured. The volume of the tank is to be determined.
Analysis The mass of the refrigerant contained in the tank is
/kgm 0.0008580
m 0.03
3
3
1
1
v
V
since
R-134a