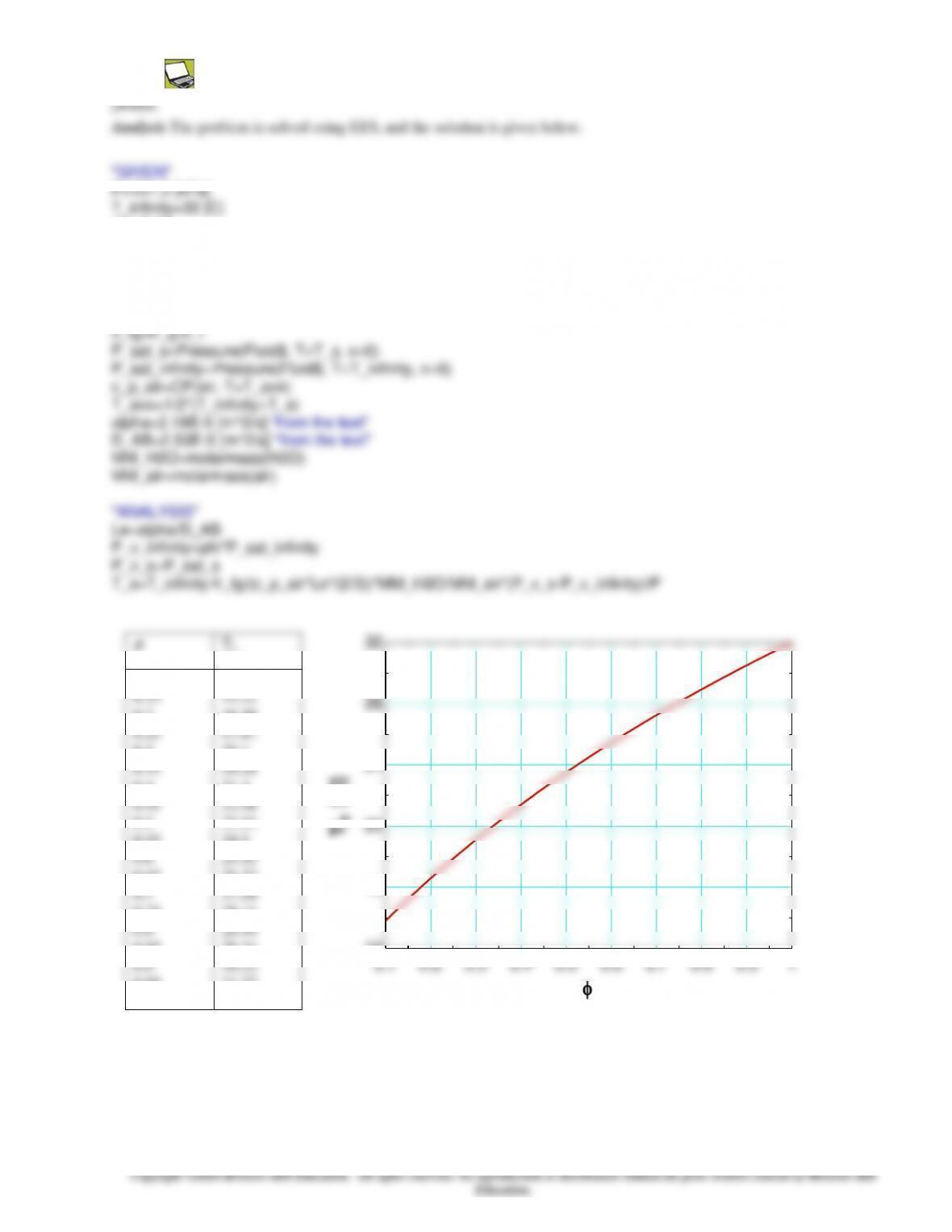
14-164 Glass bottles are washed in hot water in an uncovered rectangular glass washing bath. The rates of heat loss from the
top and side surfaces of the bath by radiation, natural convection, and evaporation as well as the rates of heat and water mass
that need to be supplied to the water are to be determined.
Assumptions 1 The low mass flux conditions exist so that the
Chilton-Colburn analogy between heat and mass transfer is
negligible. 5 The air motion around the bath is negligible so that
there are no forced convection effects.
Properties The air-water vapor mixture is assumed to be dilute,
and thus we can use dry air properties for the mixture at the
( )
/sm1073.2
atm1
K5.310
1087.11087.1 25
072.2
10
072.2
10
air-OH2
−−− ==== P
T
DD AB
The saturation pressure of water at 25C is
Properties of water at 50C are
kPa 35.12 and kJ/kg 2383 == vfg Ph
(Table A-9). The specific heat of water at the average temperature of (15+50)/2 =
32.5C is cBpB = 4.178 kJ/kg.C.
The gas constants of dry air and water are RBairB = 0.287 kPa.mP3P/kg.K and RBwaterB = 0.4615 kPa.mP3P/kg.K (Table
A-1). Also, the emissivities of water and the sheet metal are given to be 0.61 and 0.95, respectively, and the specific heat of
glass is given to be 1.0 kJ/kg.C.
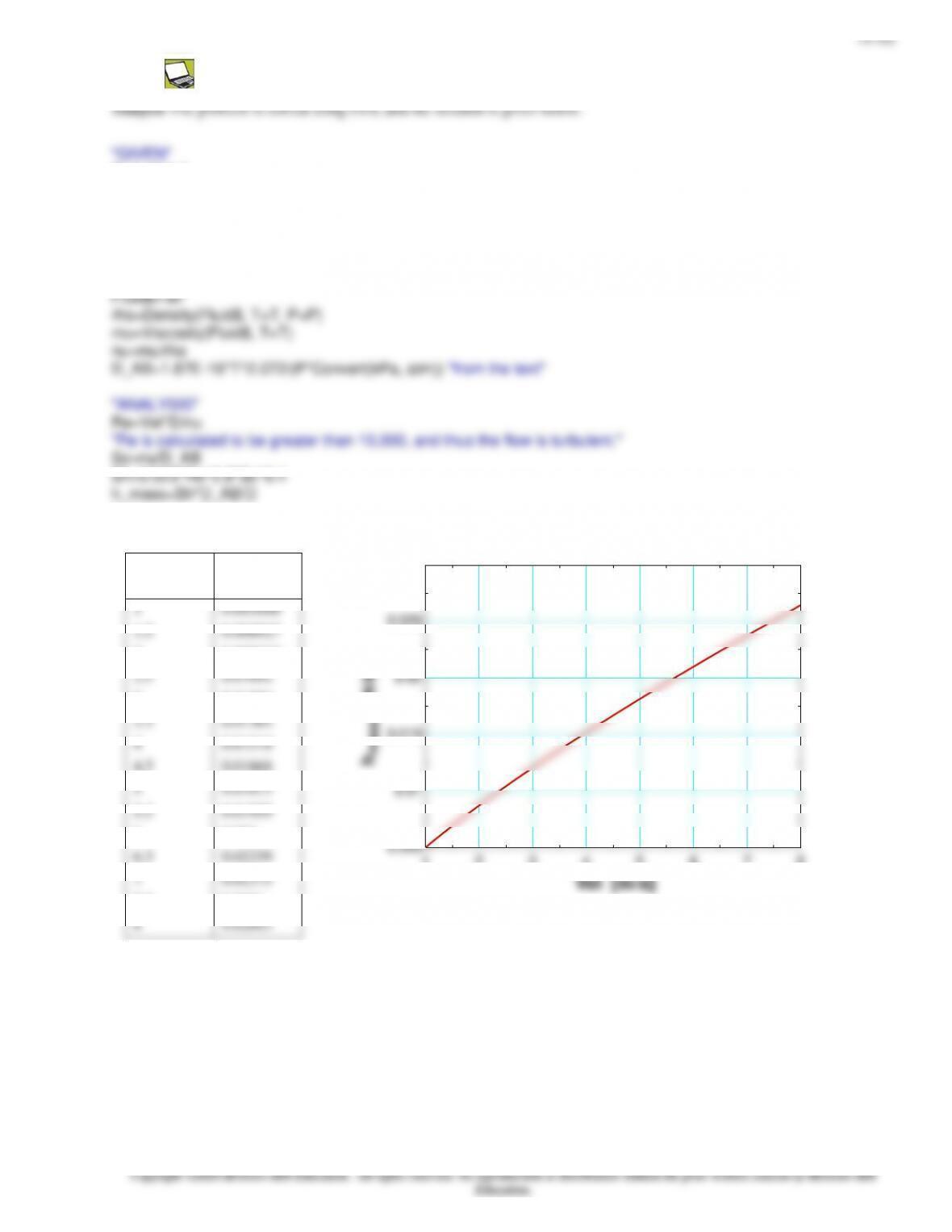
Analysis (a) The mass flow rate of glass bottles through the water bath in steady operation is
kg/s 2=kg/min 120=n)bottles/mi (800kg/bottle) (0.150=rate flow Bottle
bottlebottle = mm