14-116EE The pressure in a helium pipeline is maintained constant by venting to the atmosphere through a long tube. The
mass flow rates of helium and air, and the net flow velocity at the bottom of the tube are to be determined.
Assumptions 1 Steady operating conditions exist. 2 Helium and atmospheric air are ideal gases. 3 No chemical reactions
occur in the tube. 4 Air concentration in the pipeline and helium concentration in the atmosphere are negligible so that the
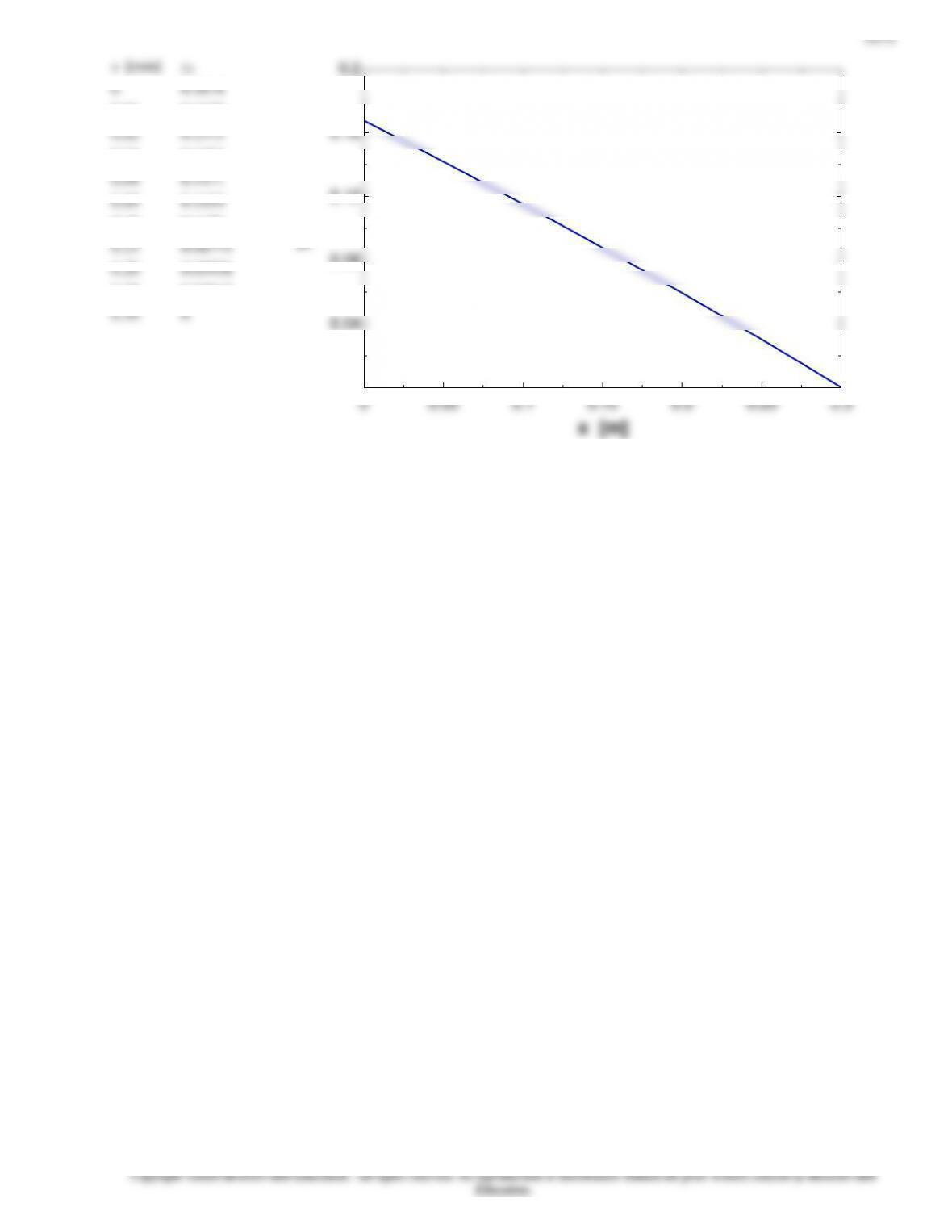
Noting that the pressure of helium is 14.5 psia at the bottom of the tube
(x = 0) and 0 at the top (x = L), its molar flow rate is
lbmol/s 10642.5
ft 30
psia )05.14(
R) 540/lbmol.R)(psia.ft (10.73
)ft 10727.8(/s)ft 1075.7(
11
3
2424
,0,
Adiff,helium
−
−−
=
−
=
−
== L
PP
TR
AD
NN LAA
u
AB
Therefore, the mass flow rate of helium through the tube is
lbm/s 102.26 10−− === lbm/lbmol) lbmol/s)(4 10642.5()( 11
heliumhelium MNm
which corresponds to 0.00712 lbm per year.
01034.2
lbm/s )1064.11026.27(
lbm/s 101.64 10
910
9
total
air
air =
+−
== −
−−
−
m
m
w
3
3
helium lbm/ft 00201.0
R) 0/lbm.R)(54psia.ft (2.681
psia 5.14 === RT
P