Answer:
How are oxygen molecules attracted to water molecules?
A) The attraction between oxygen and water molecules is a classic example of
dipole-dipole interaction.
B) The hydrogen bonding in water causes the attraction of the oxygen atoms in the
molecule to water.
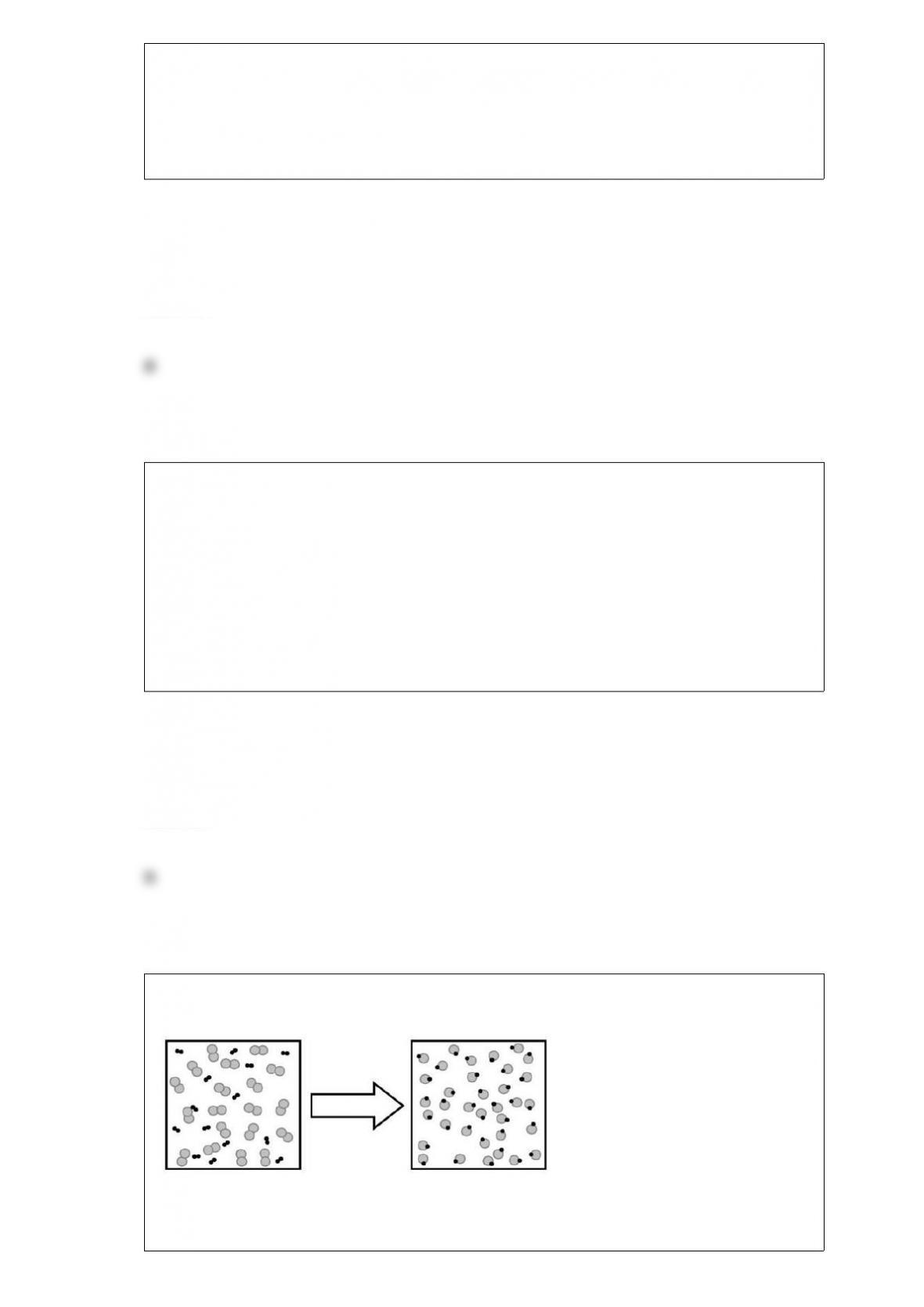
C) As a water molecule is brought close to an oxygen molecule an induced dipole
results in the molecule causing the attraction.
D) The attraction of oxygen and water molecules for one another is part of the common
atom effect. Since both molecules contain oxygen, there is a built-in attraction.
Answer:
If Burl carried Paul piggy-back while standing in the middle of a scaffold, the tensions
in the two supporting ropes would
A) cancel to zero.
B) be equal.
C) be unequal.
D) more easily support Burl and Paul.