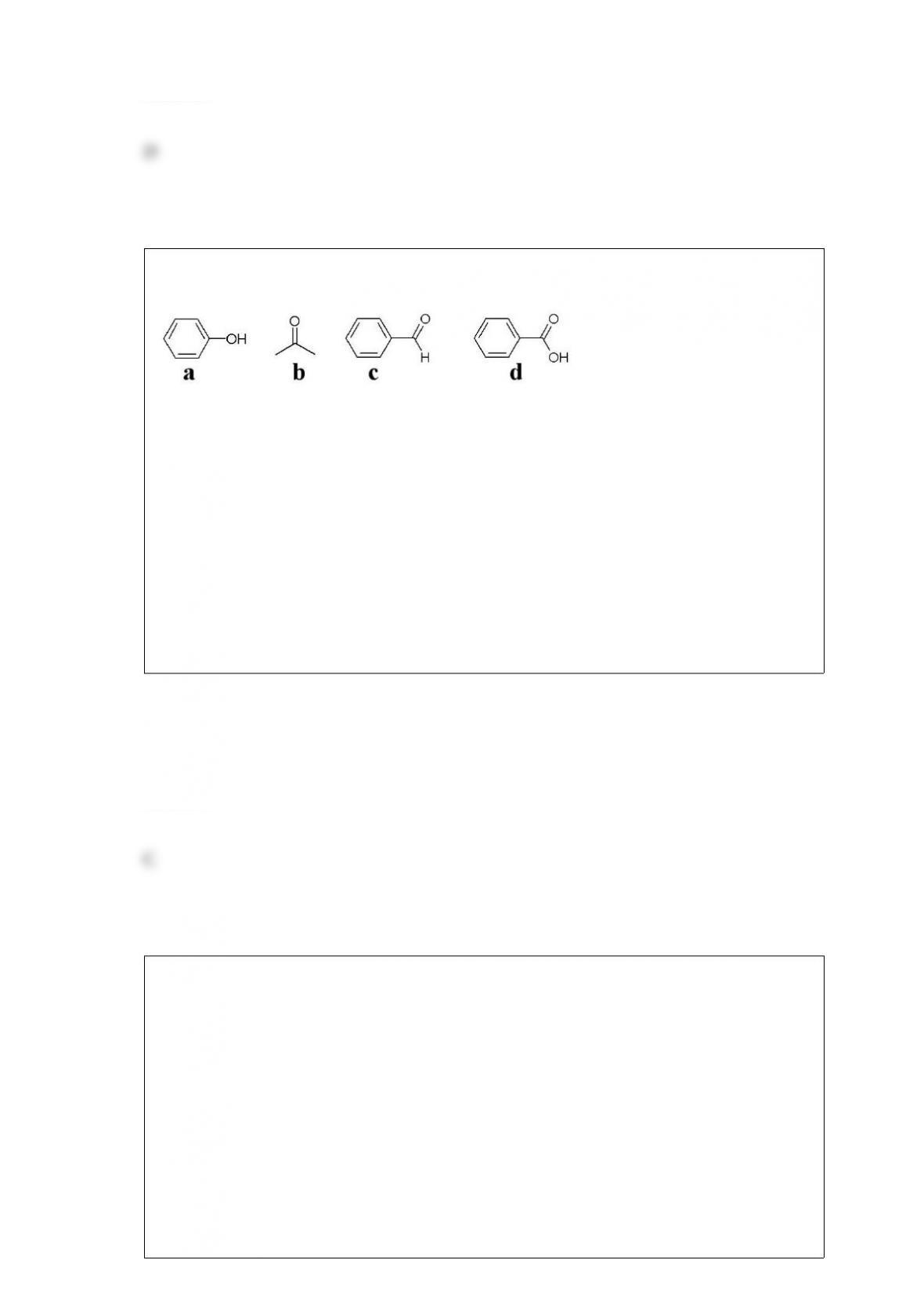
E) Light is composed of waves that exist only at certain quantum fundamental
frequencies.
Answer:
Should the periodic table be memorized? Why or why not?
A) Yes. Like the alphabet, we need to memorize the periodic table in order to easily
write the language of chemistry.
B) Yes. Without memorizing the periodic table, one would not have any real
understanding of how and why chemical compounds are put together.
C) No. The periodic table changes every year. Memorizing it would be a waste of time.
D) No. The periodic table is a reference to be used, not memorized.
Answer:
The lithosphere includes
A) continental and oceanic crust.
B) the crust and the upper part of the mantle.
C) part of the mantle and the crust.
D) continental and oceanic crust and the upper part of the mantle.