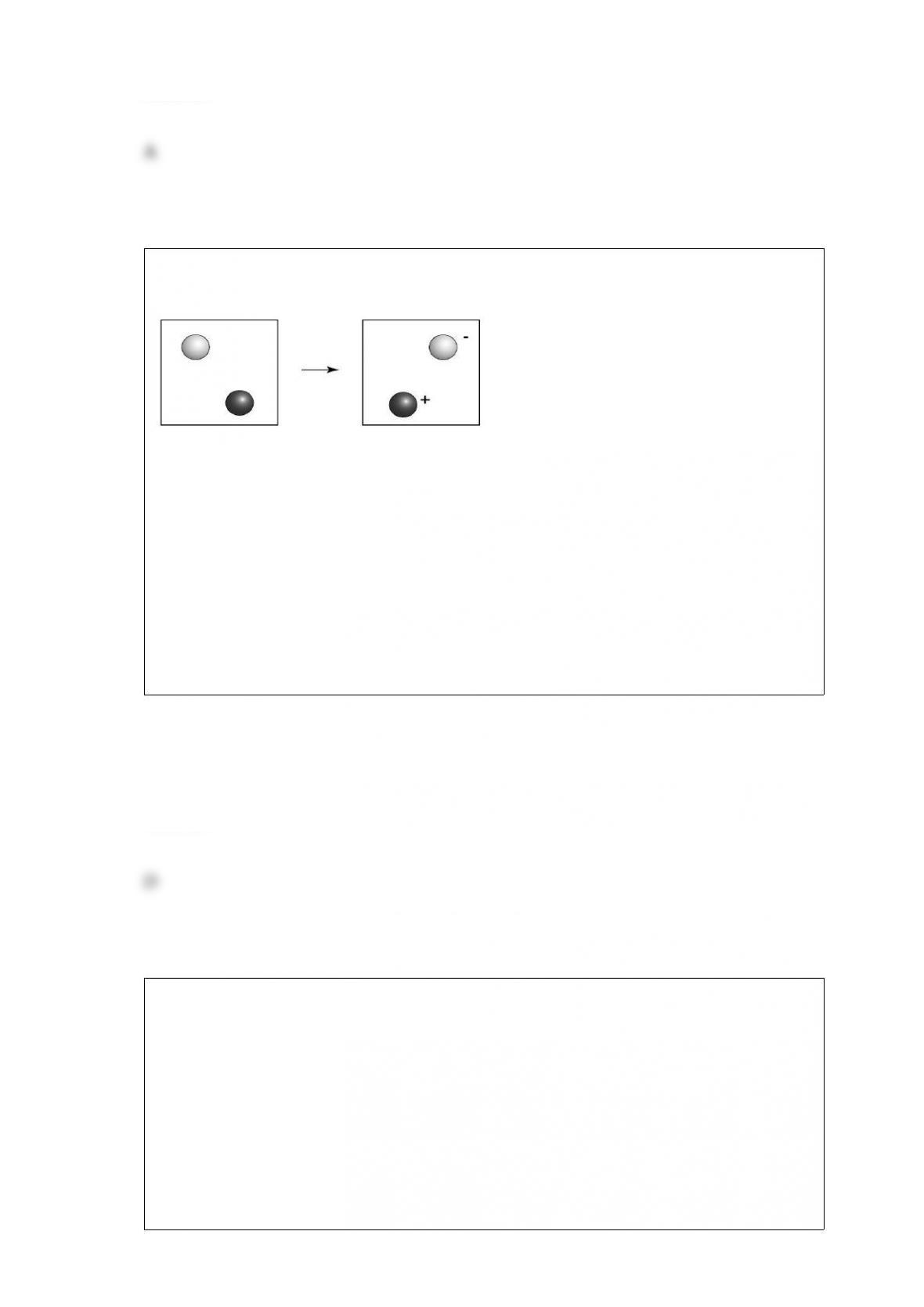
E) none of the above
Answer:
Someone argues that he or she doesn't drink tap water because it contains thousands of
molecules of some impurity in each glass. How would you respond in defense of the
water's purity, if it indeed does contain thousands of molecules of some impurity per
glass?
A) Impurities aren't necessarily bad, in fact, they may be good for you.
B) The water contains water molecules and each water molecule is pure.
C) There's no defense. If the water contains impurities it should not be drunk.
D) Compared to the billions and billions of water molecules, a thousand molecules of
something else is practically nothing.
Answer:
Which of the following intermolecular forces best describes why molecules like sucrose
(which has many OH groups) are very water soluble?
A) dipole-dipole
B) induced dipole-induced dipole
C) dipole-induced dipole
D) ion-dipole