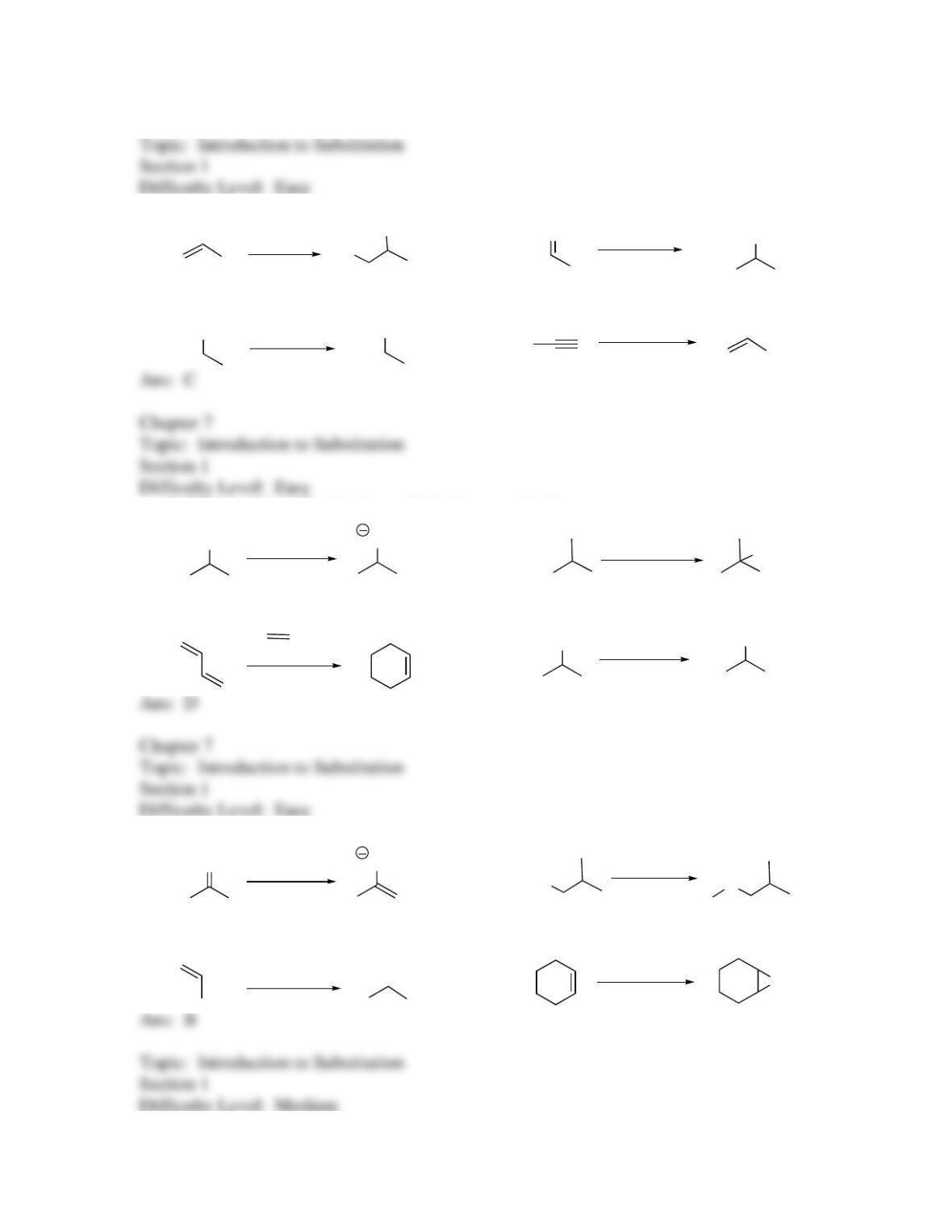
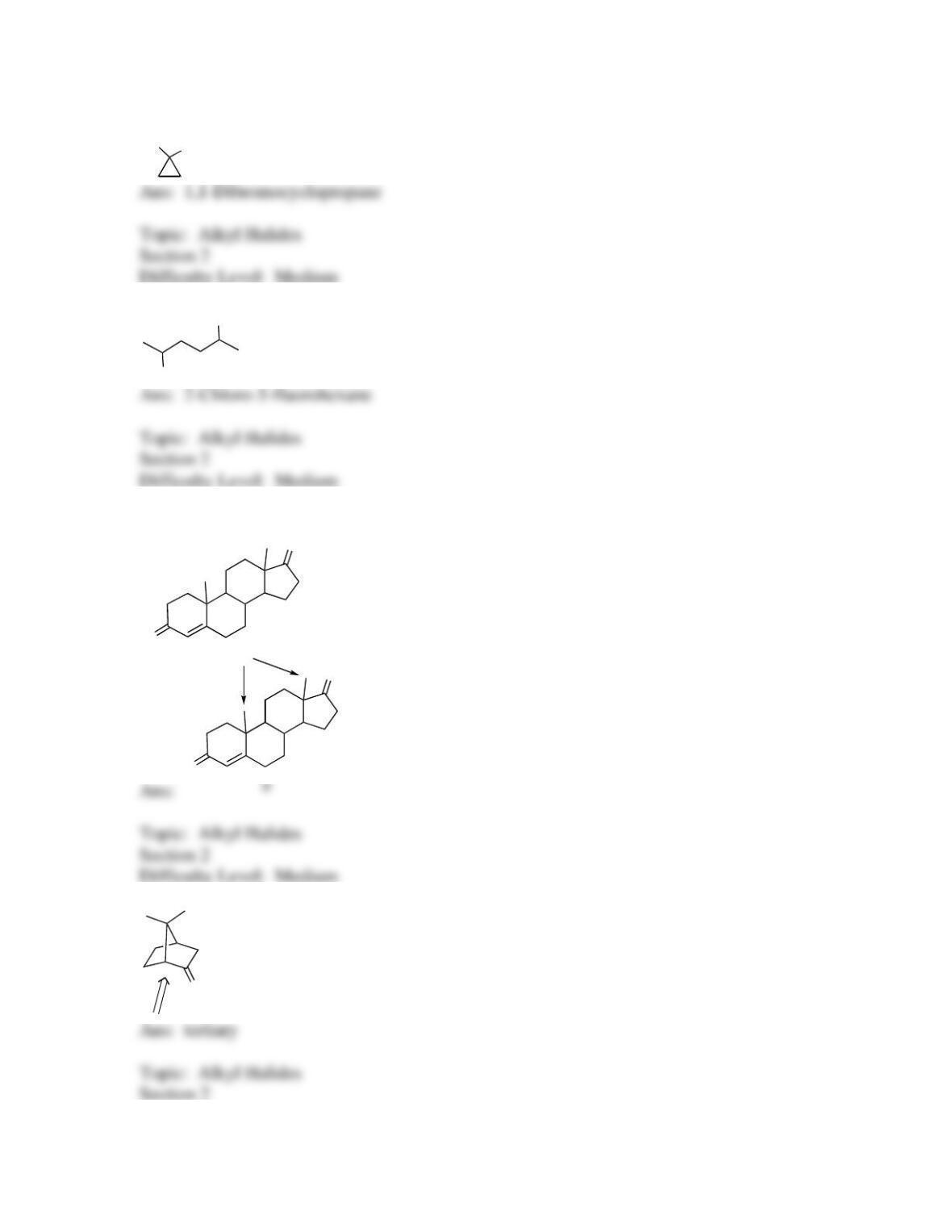
25. Identify the labeled carbon as primary, secondary, or tertiary.
26. Identify the labeled carbon as primary, secondary, or tertiary.
27. Provide a definition of a concerted reaction.
28. Which of the following is a reasonable definition of a concerted reaction?
A. It is a reaction which takes place in a series of steps.
B. It is a reaction which produces a loud noise.
C. It is a reaction in which all bond-breaking and bond-forming occurs at the same
time.
D. It is a substitution reaction.
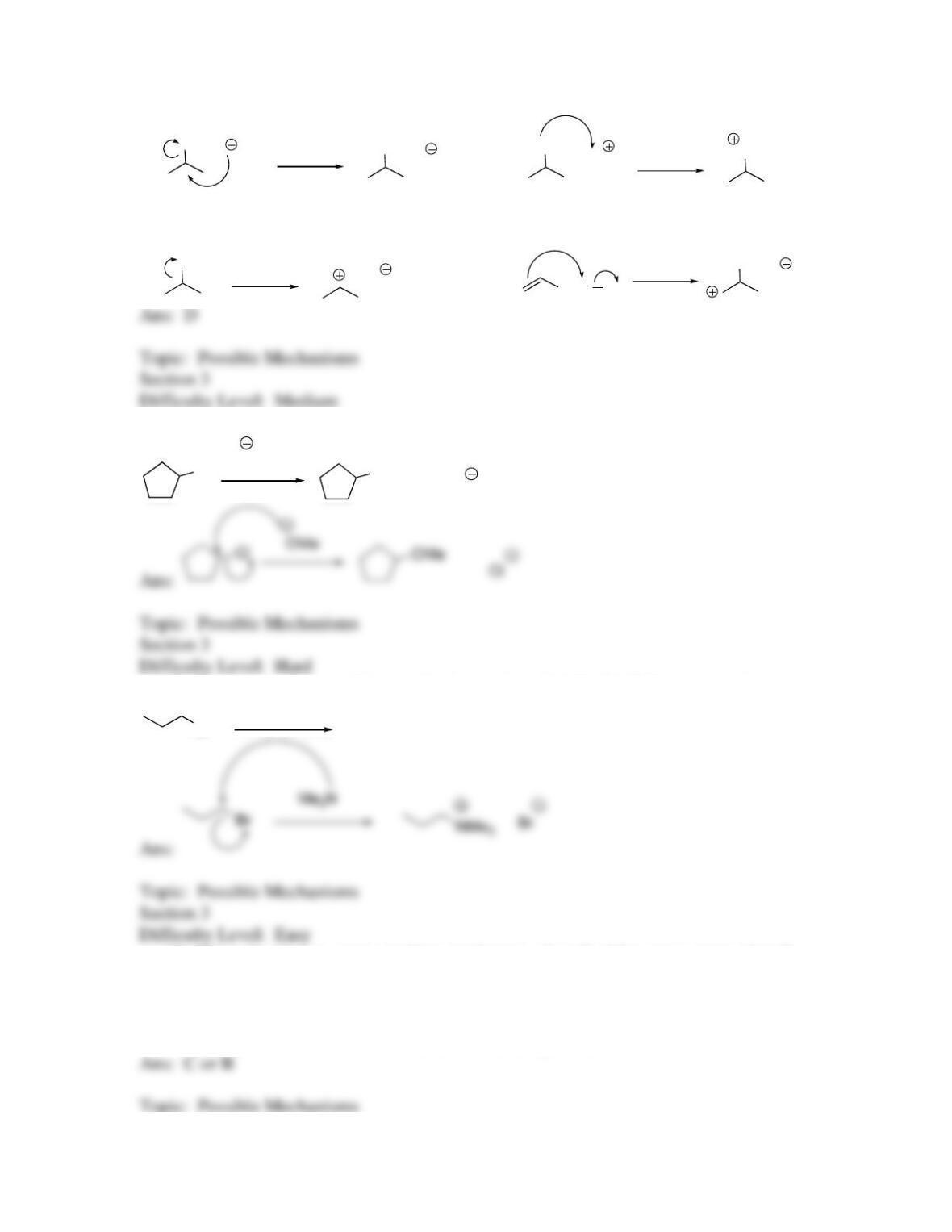
29. Provide examples of the 4 patterns for steps in the mechanisms of substitution
reactions.
30. Which of the following is not a possible step in a substitution reaction?