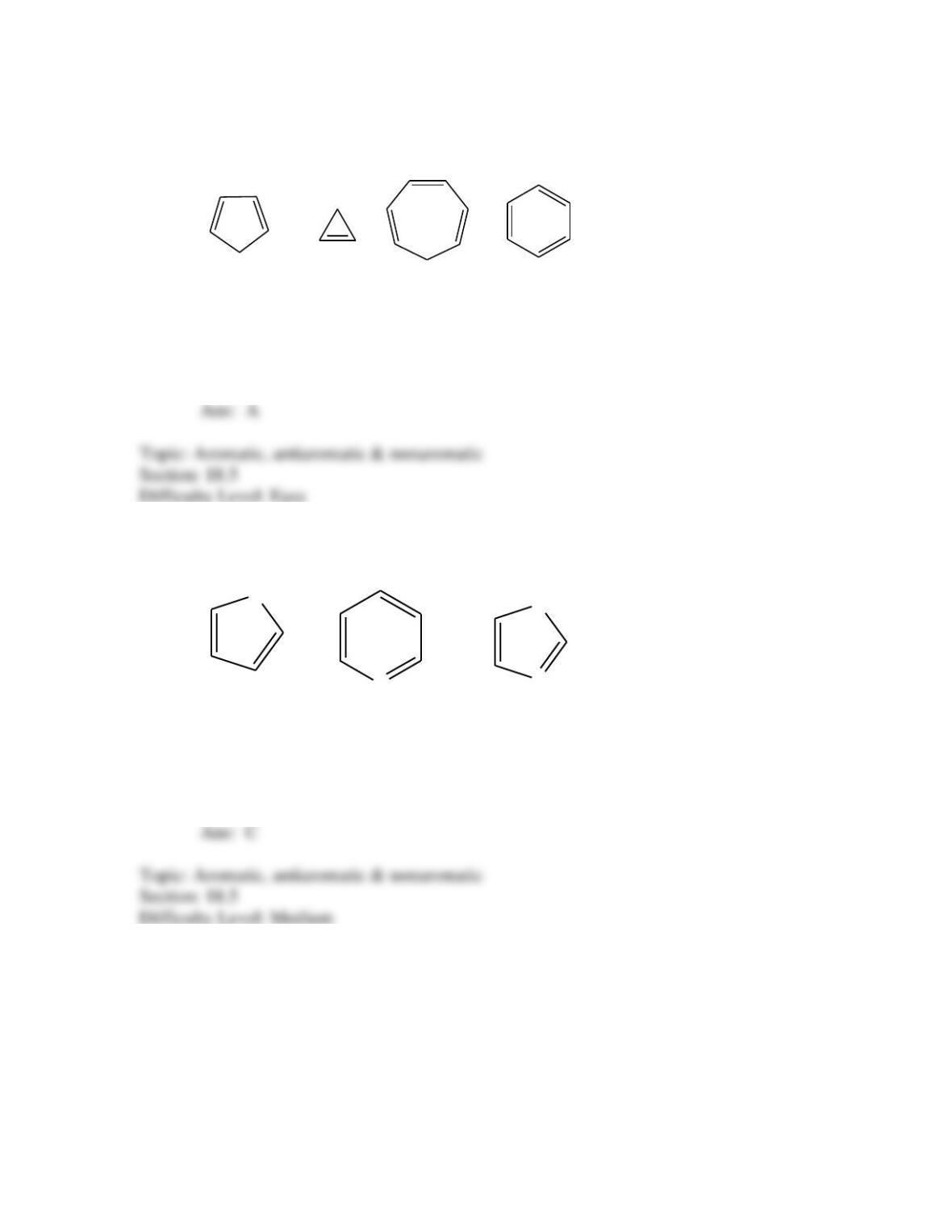
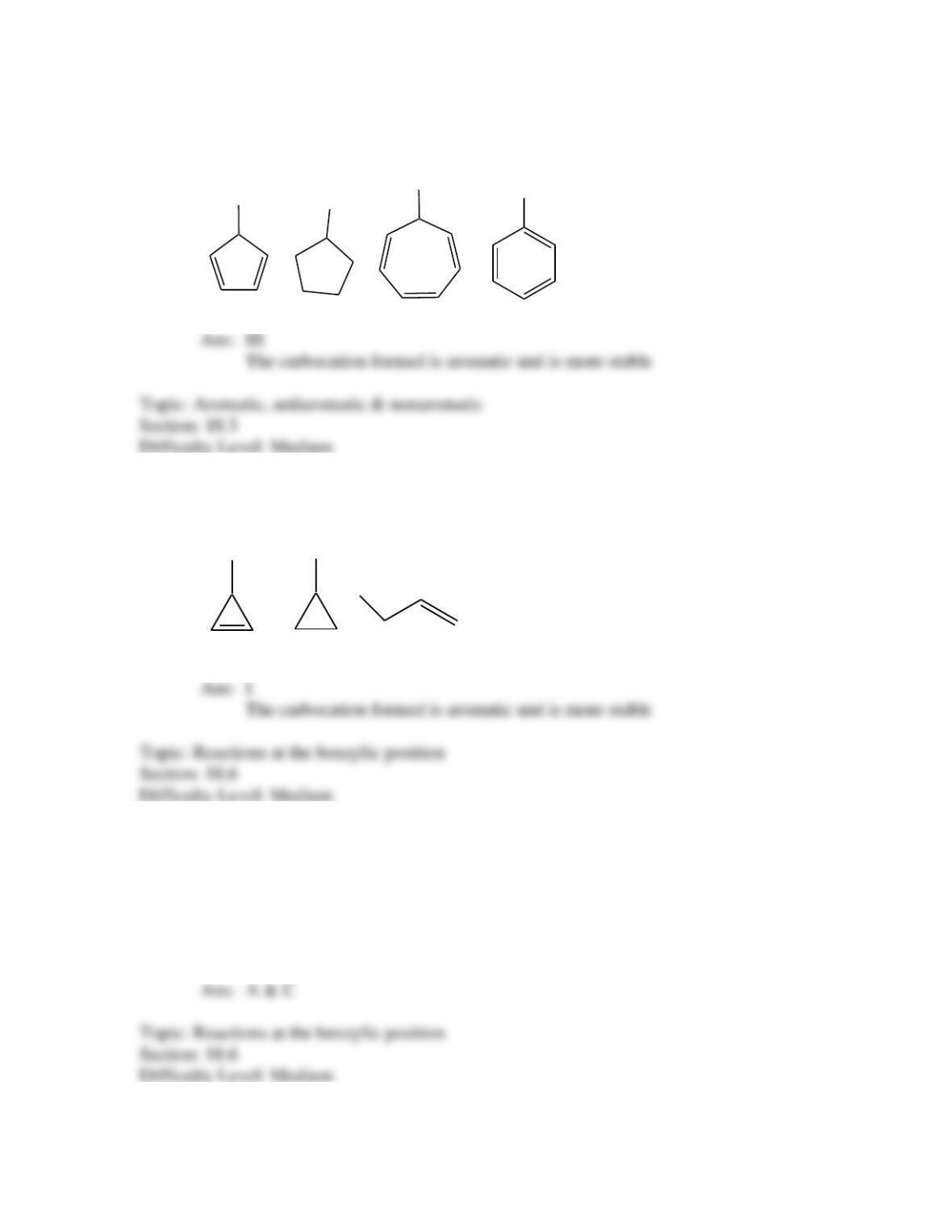
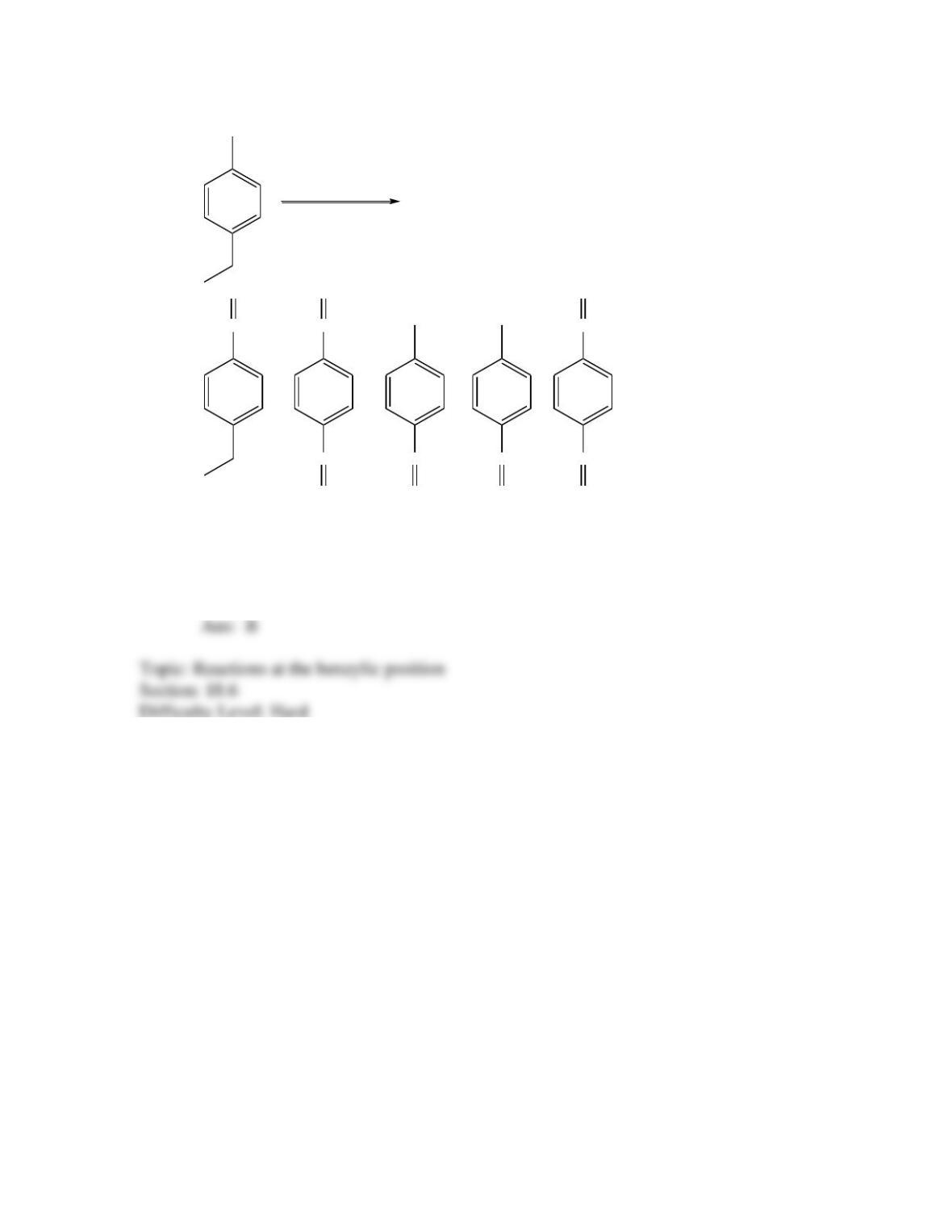
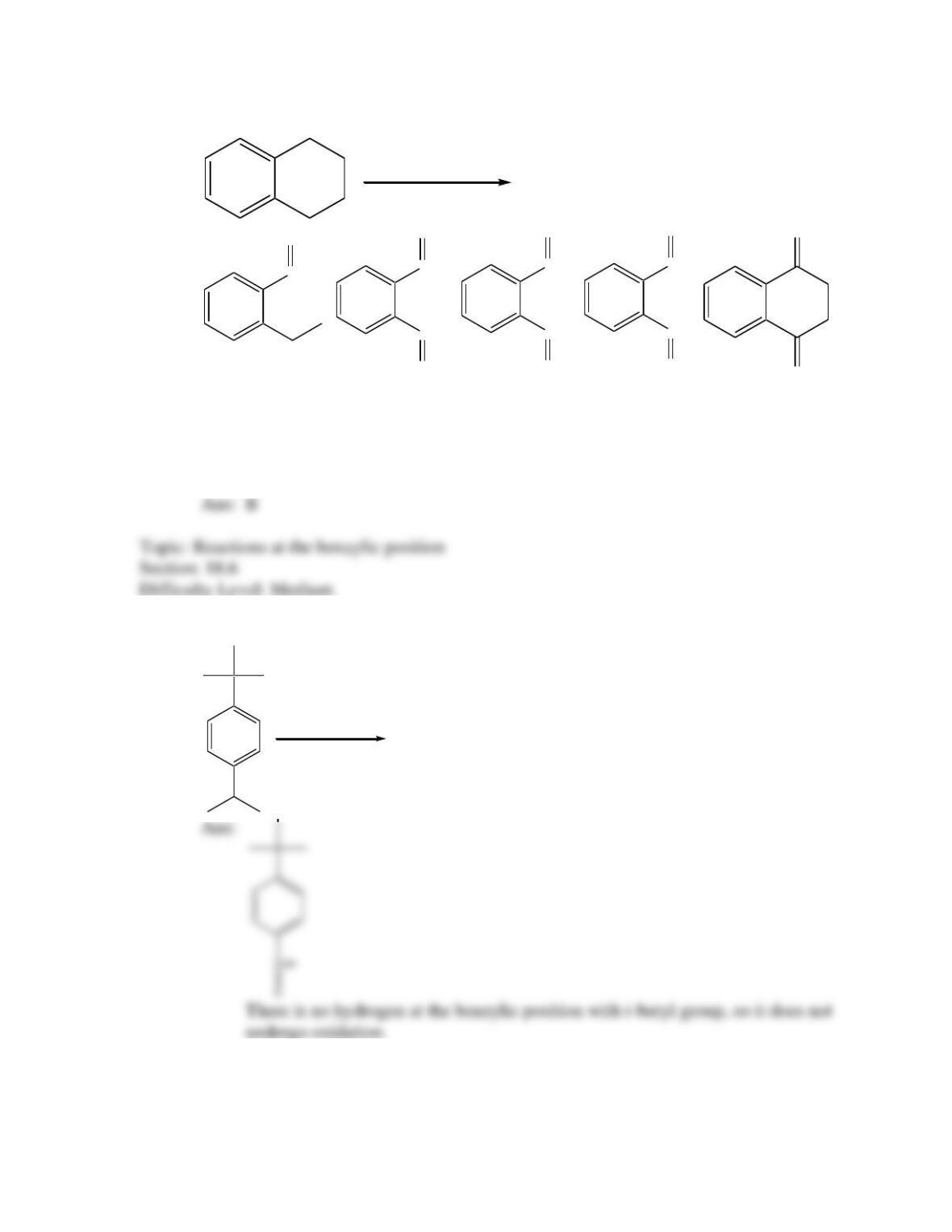
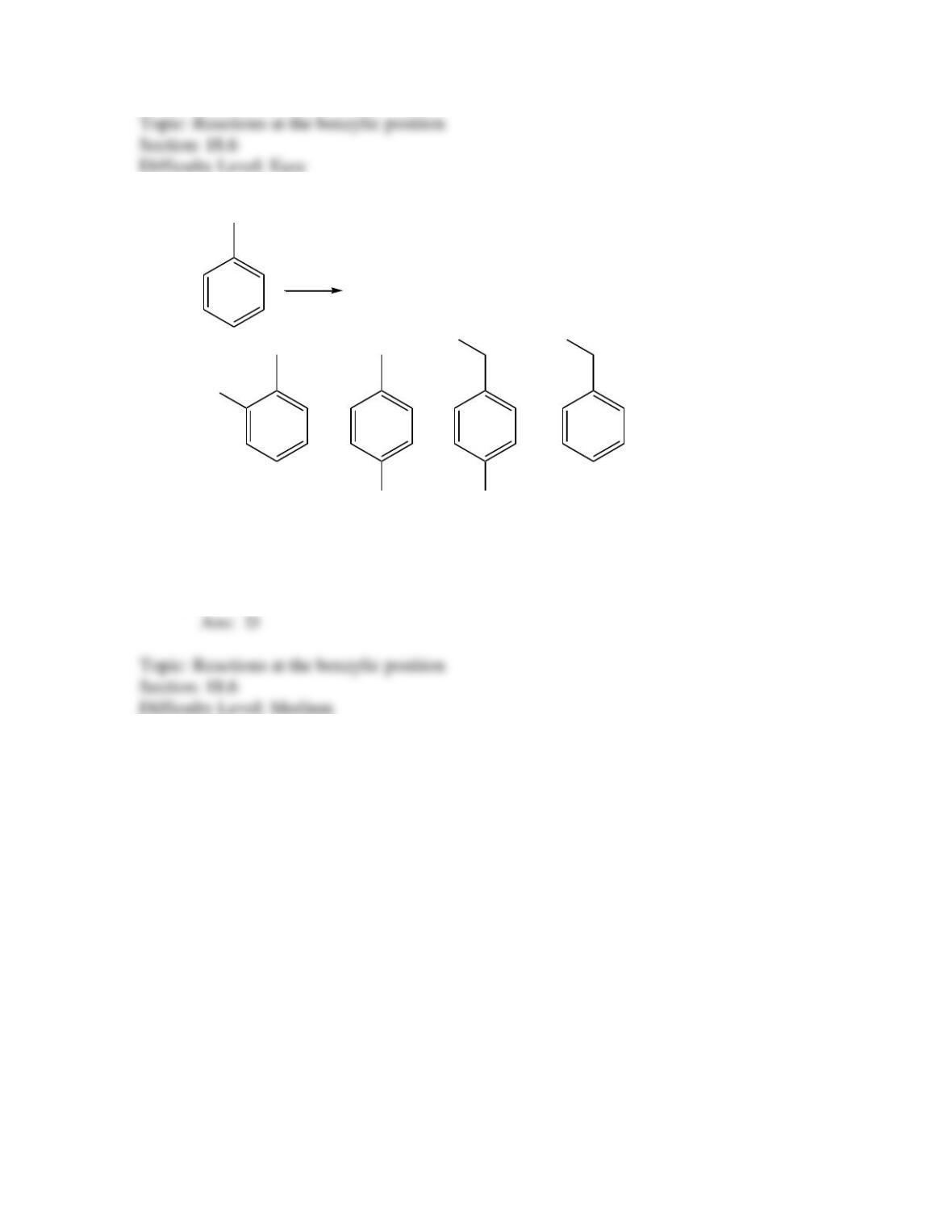
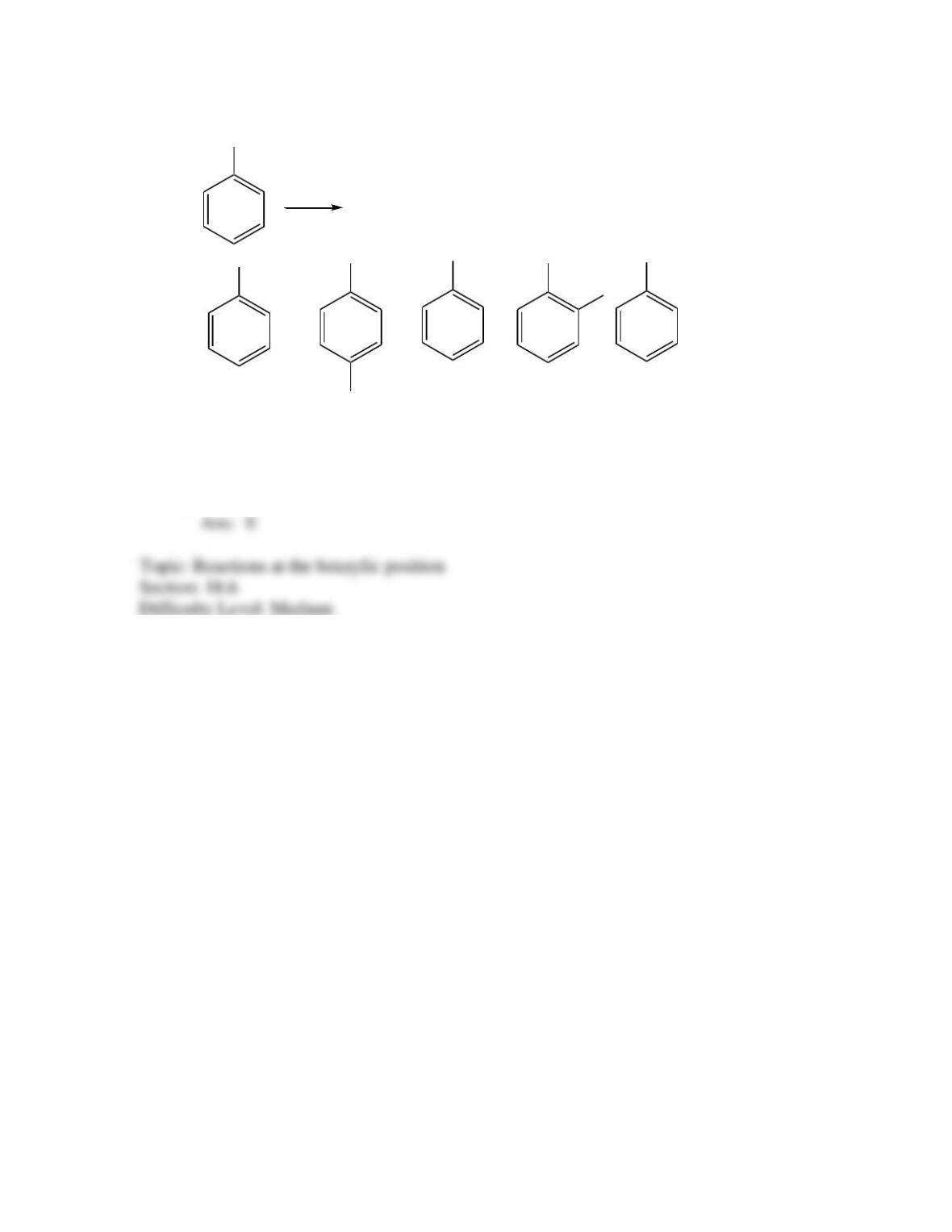
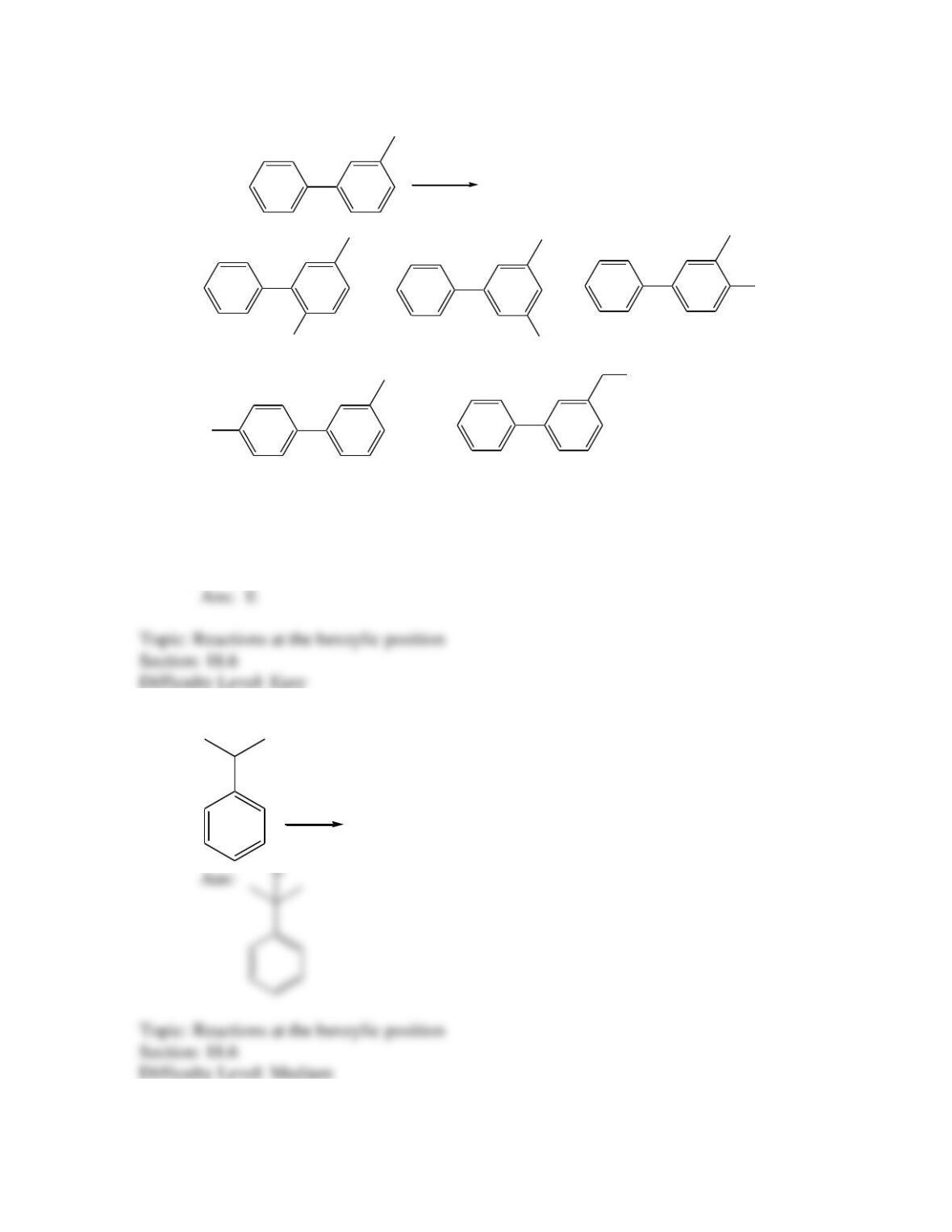
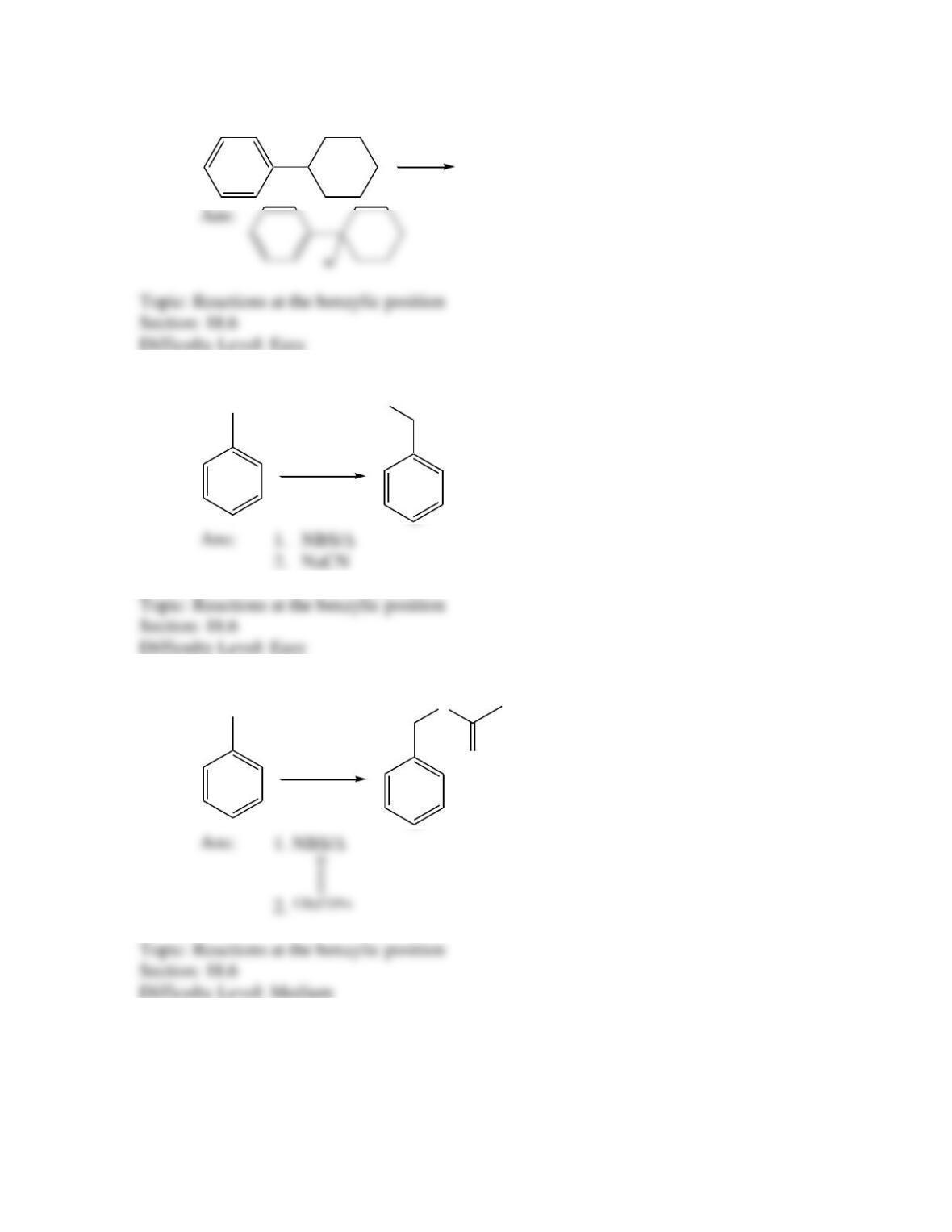
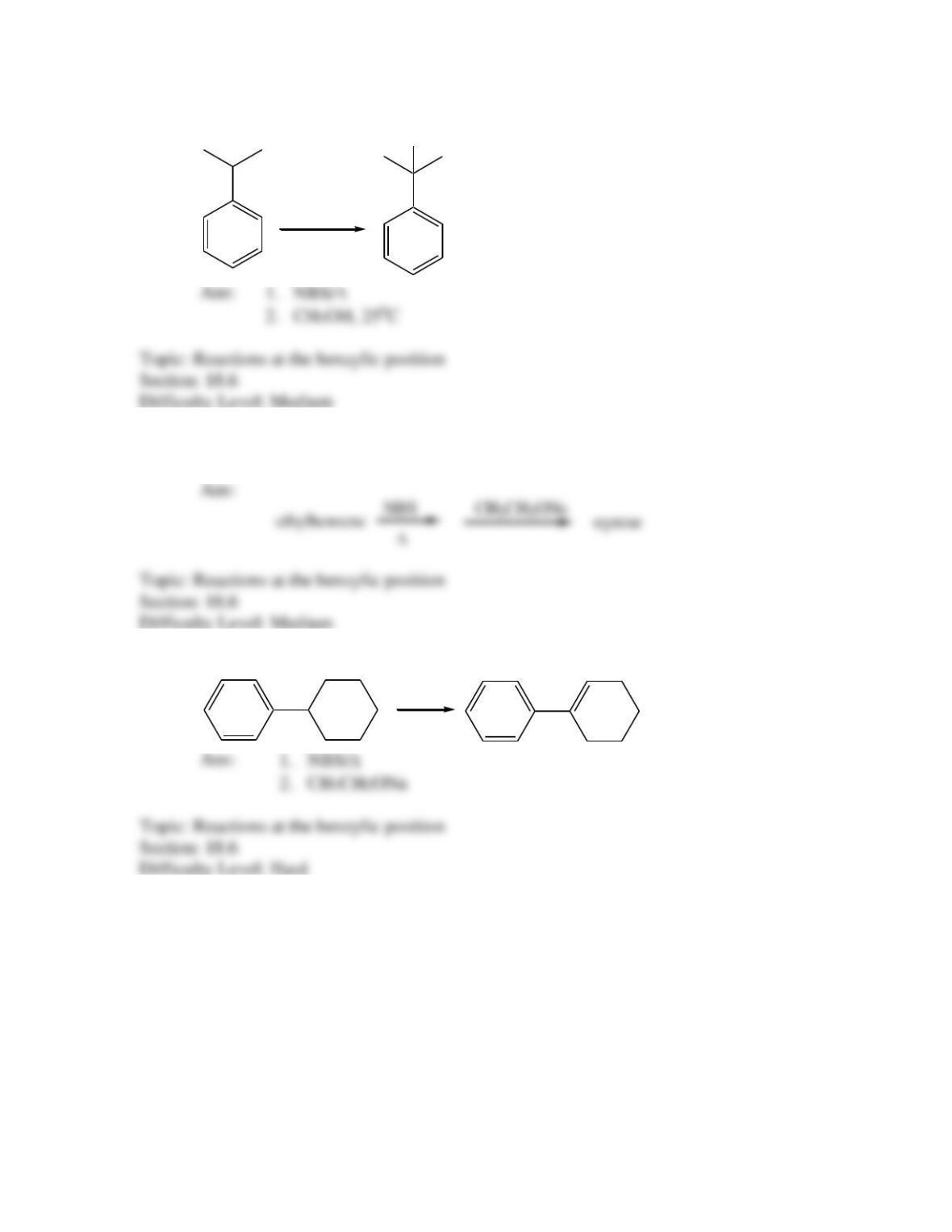
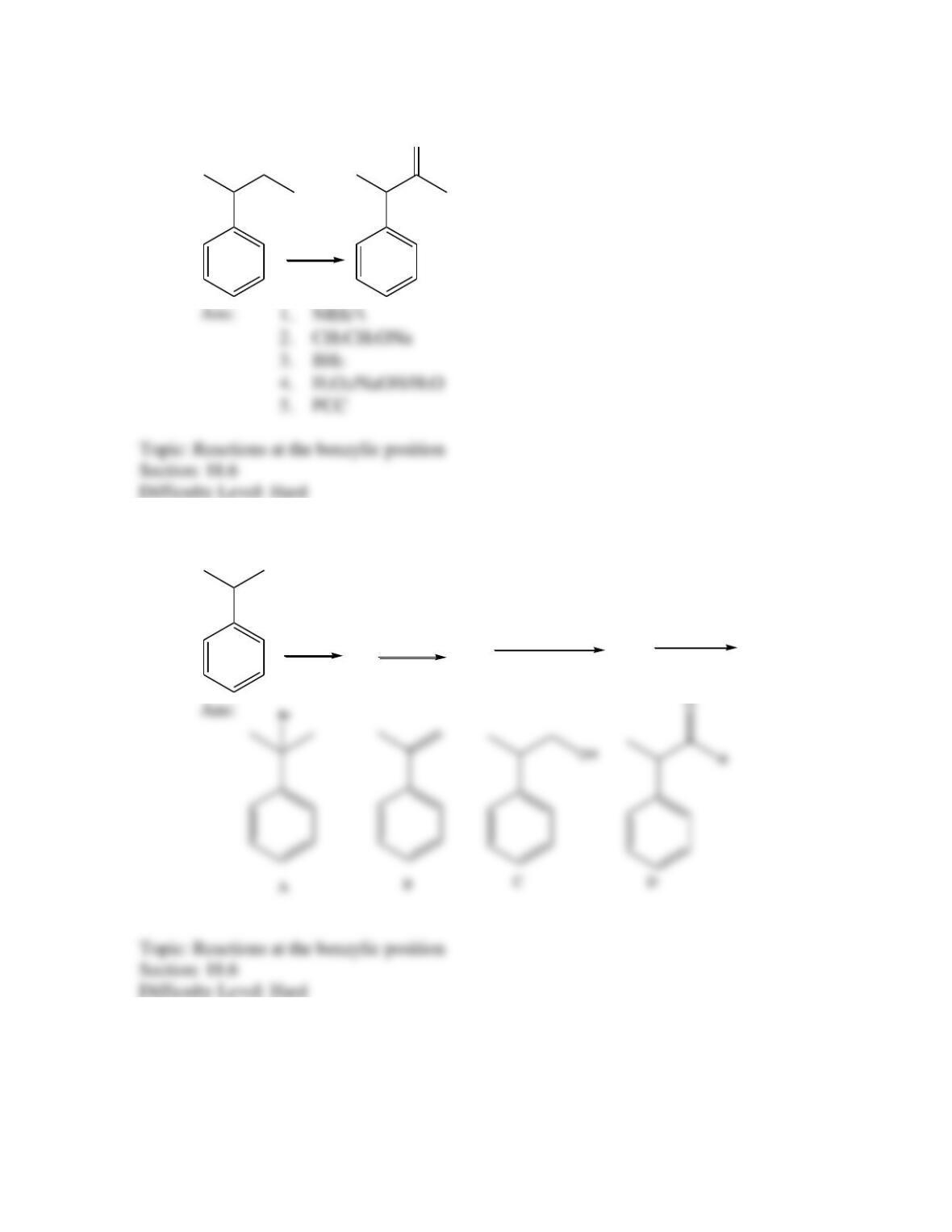
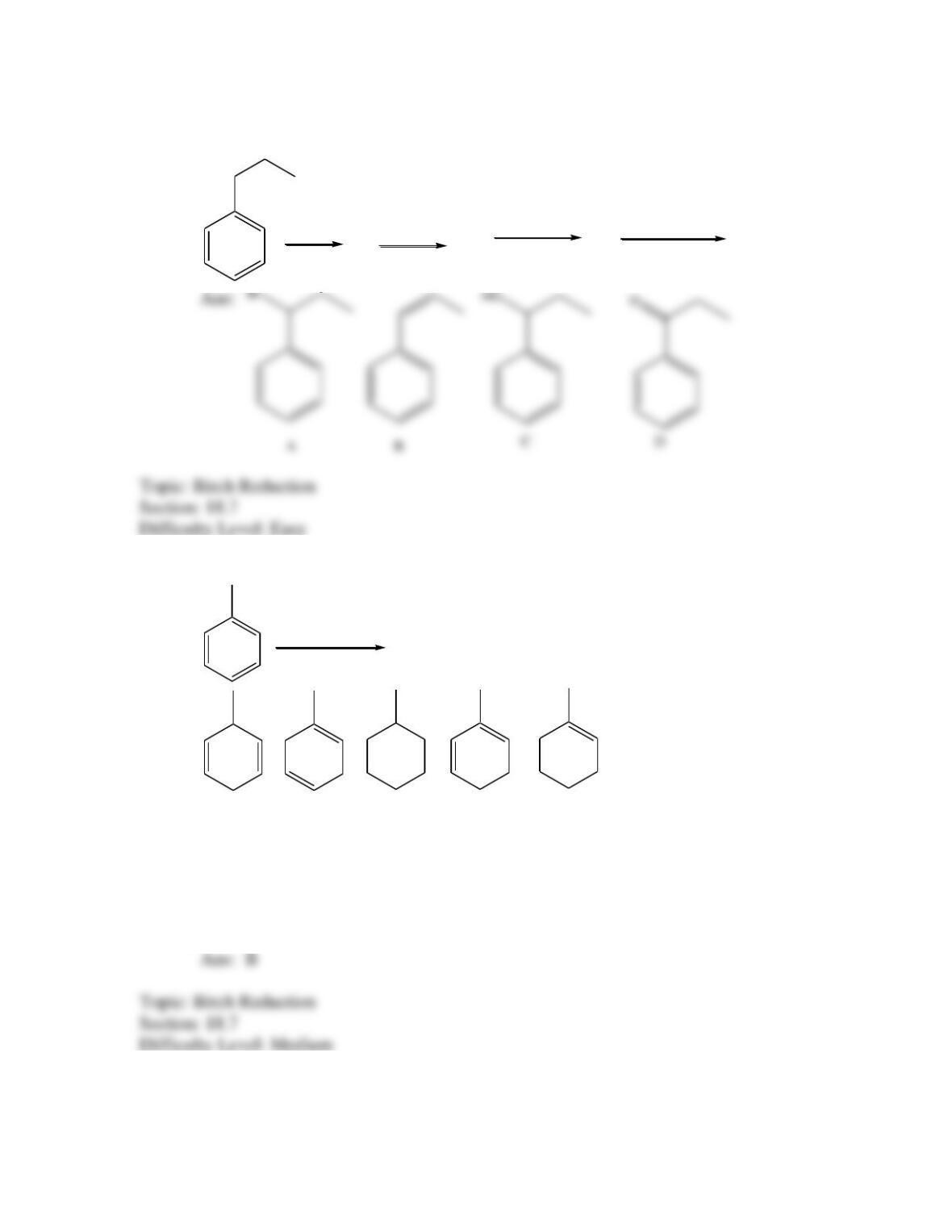
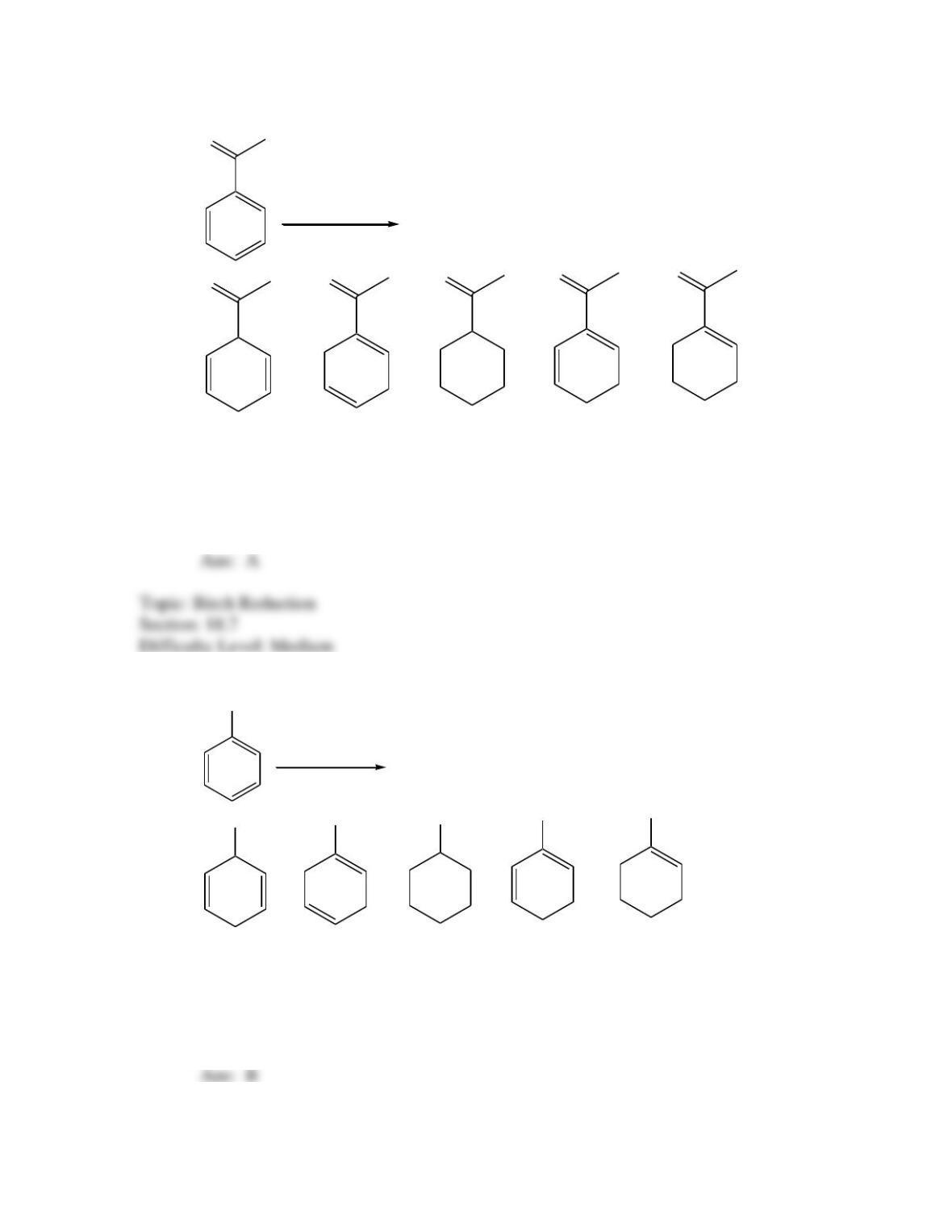
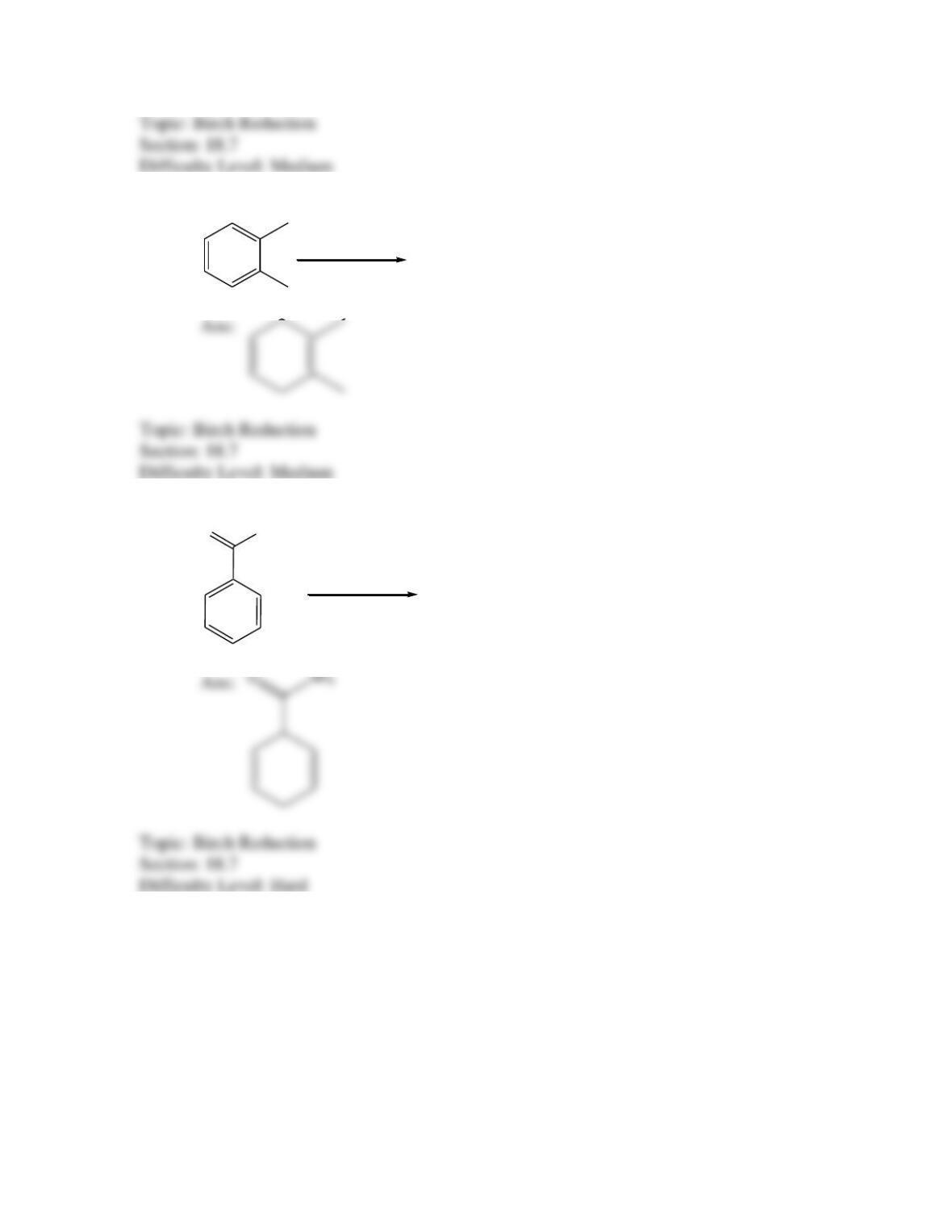
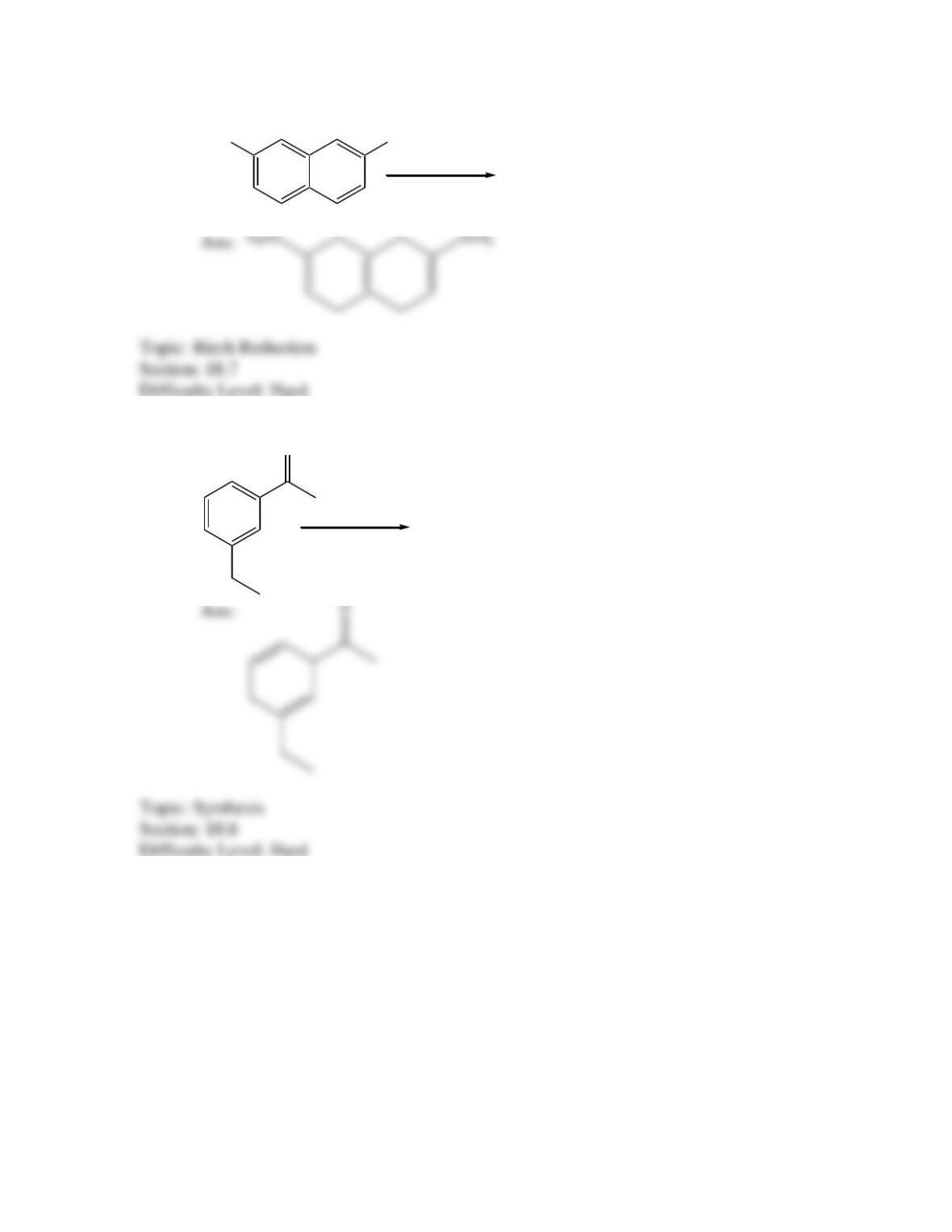
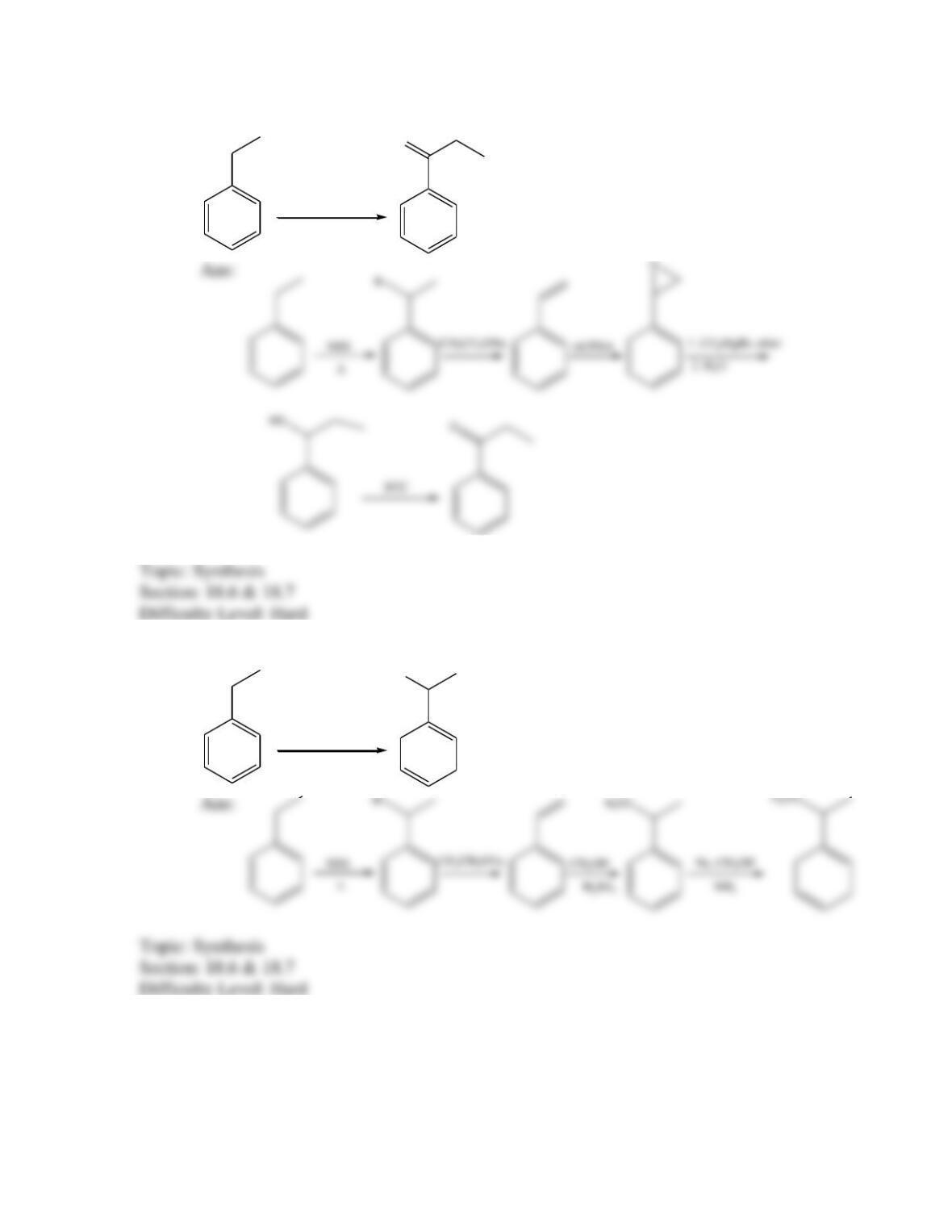
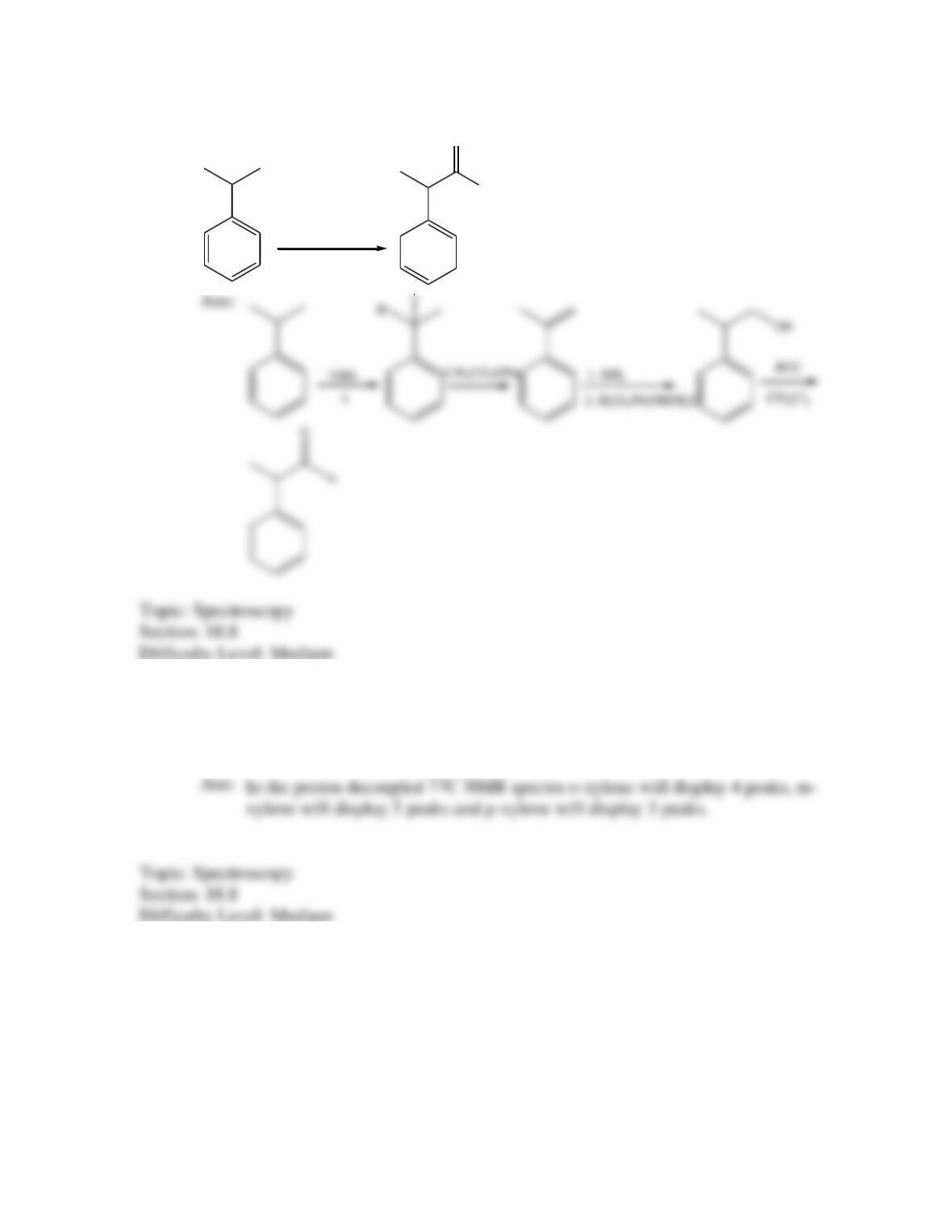
Provide a structure for the compound with molecular formula C9H9ClO and with the
following spectroscopic data.
IR: 1680 cm−1
1H NMR: 3.5 (triplet, I=2H), 4.0 (triplet, I=2H), 7.4 (triplet, I=2H), 7.6 (doublet,
I=1H), 7.9 (triplet, I=2H)
Provide a structure for the compound with molecular formula C9H12 and with the
following spectroscopic data.
1H NMR: 1.2 (doublet, I=6H), 3.0 (septet, I=1H), 7.1 (singlet, I=5H)
Provide a structure for the compound with molecular formula C10H12O2 and with the
following spectroscopic data.
IR: 1680 cm−1, 2750 cm−1, 2850 cm−1
1H NMR: 1.1 (triplet, I=3H), 3.5 (quartet, I=2H), 4.5 (singlet, I=2H), 7.3 (doublet,
I=2H), 7.7 (doublet, I=2H), 9.9 (singlet, I=1H)