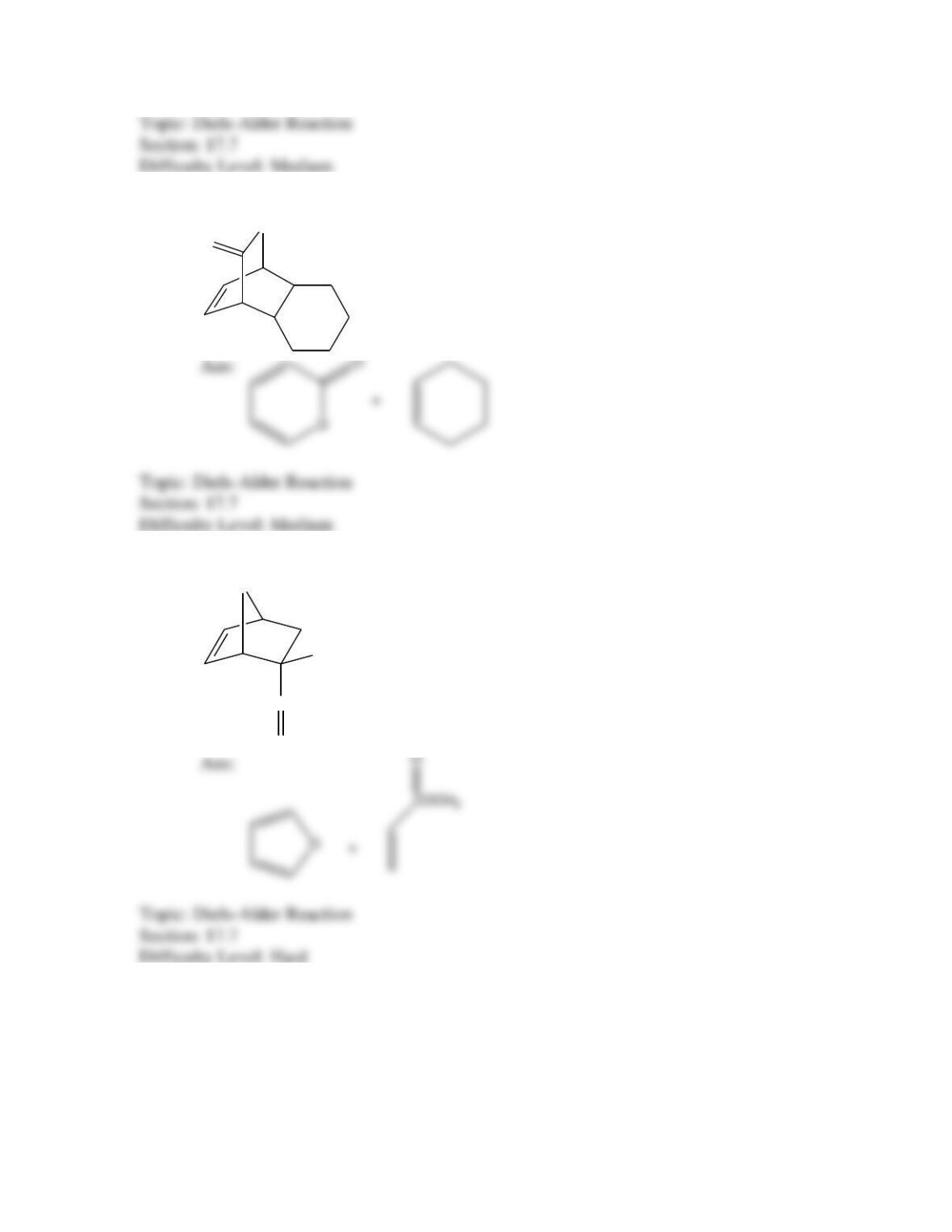
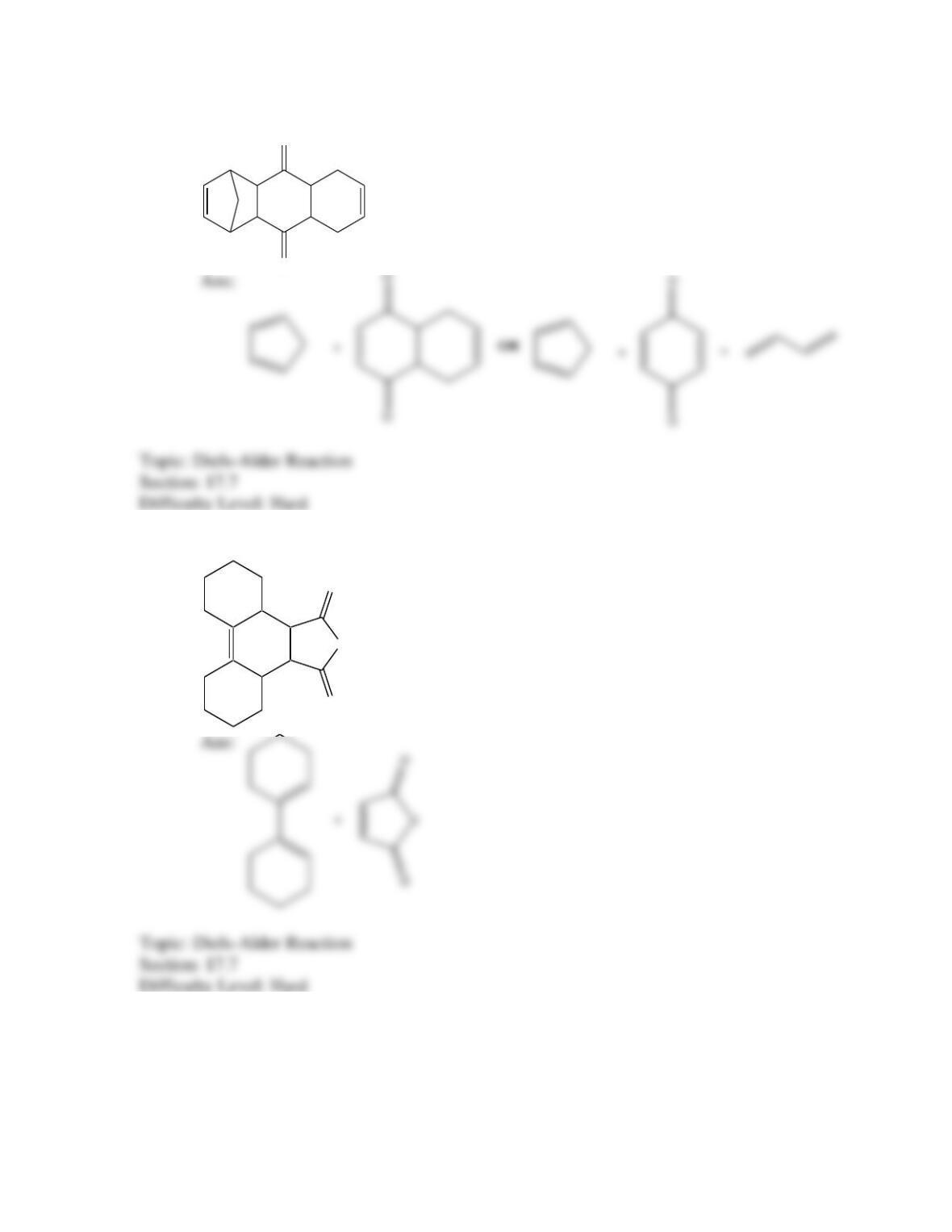
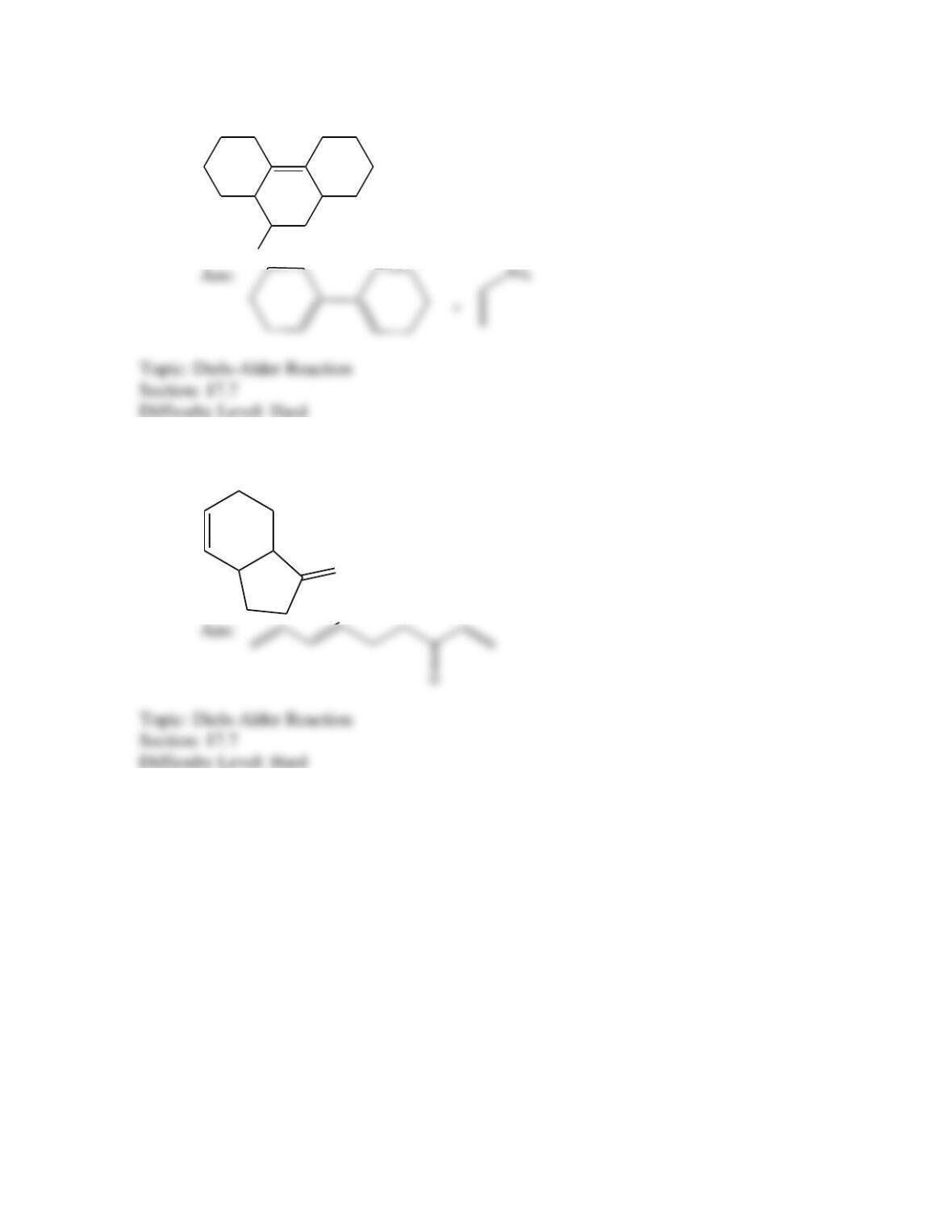
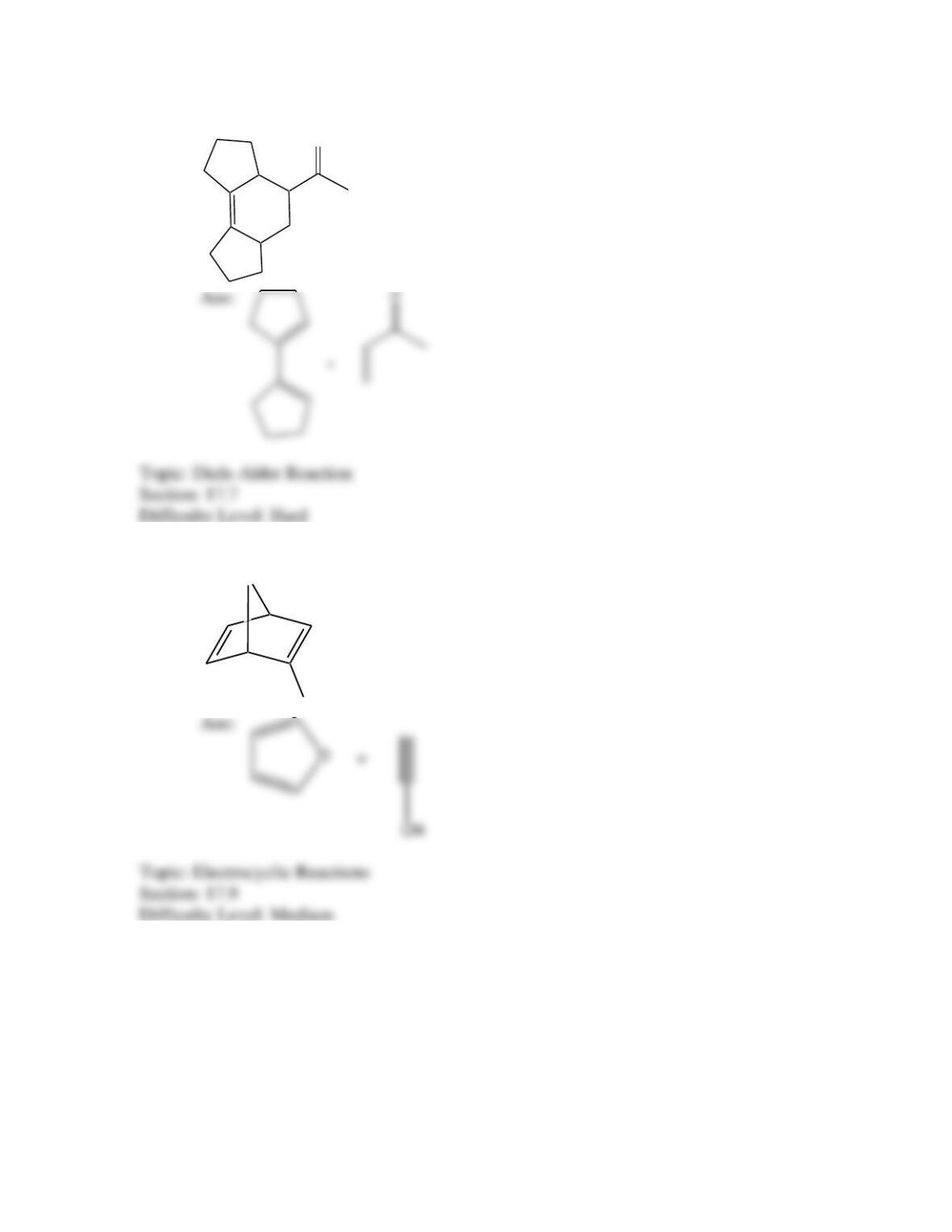
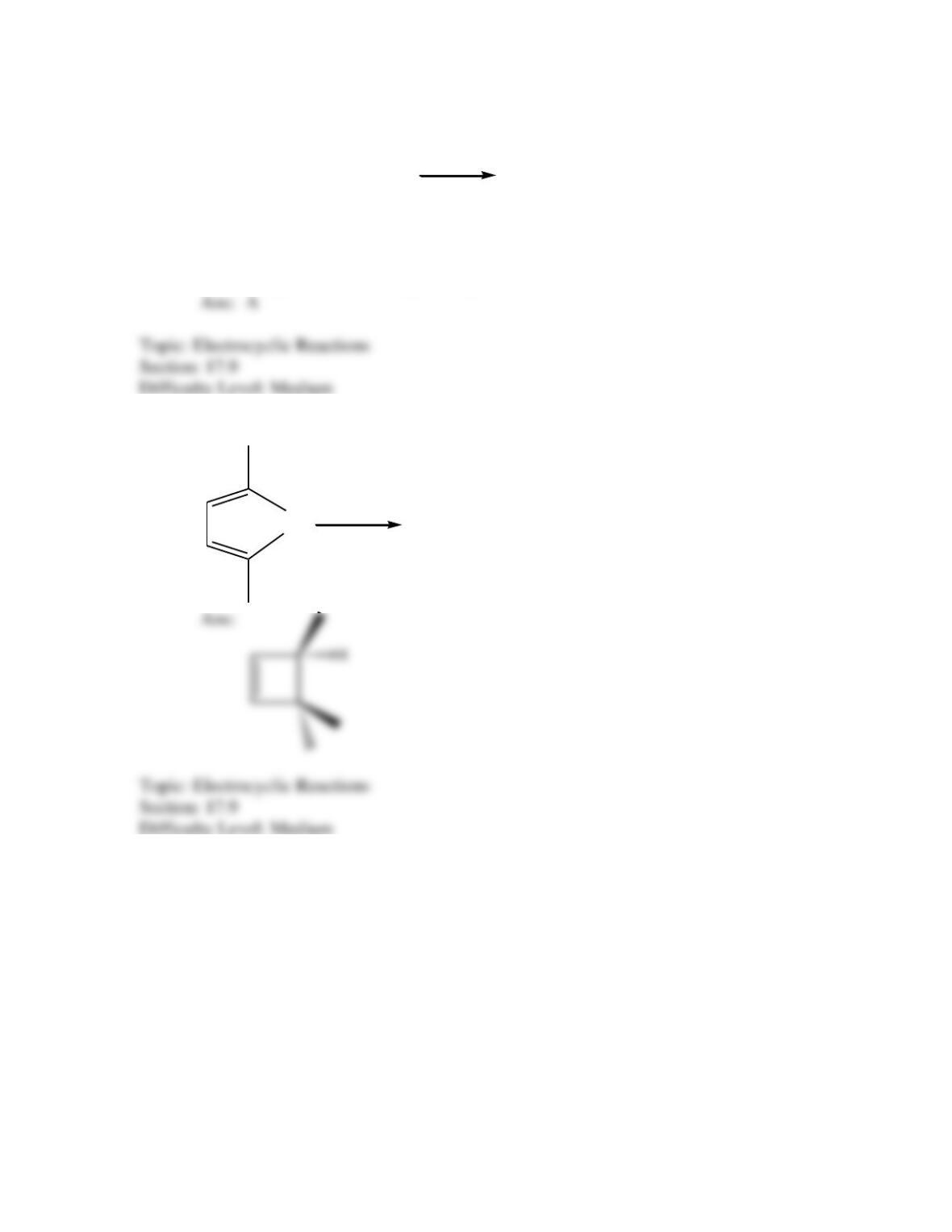
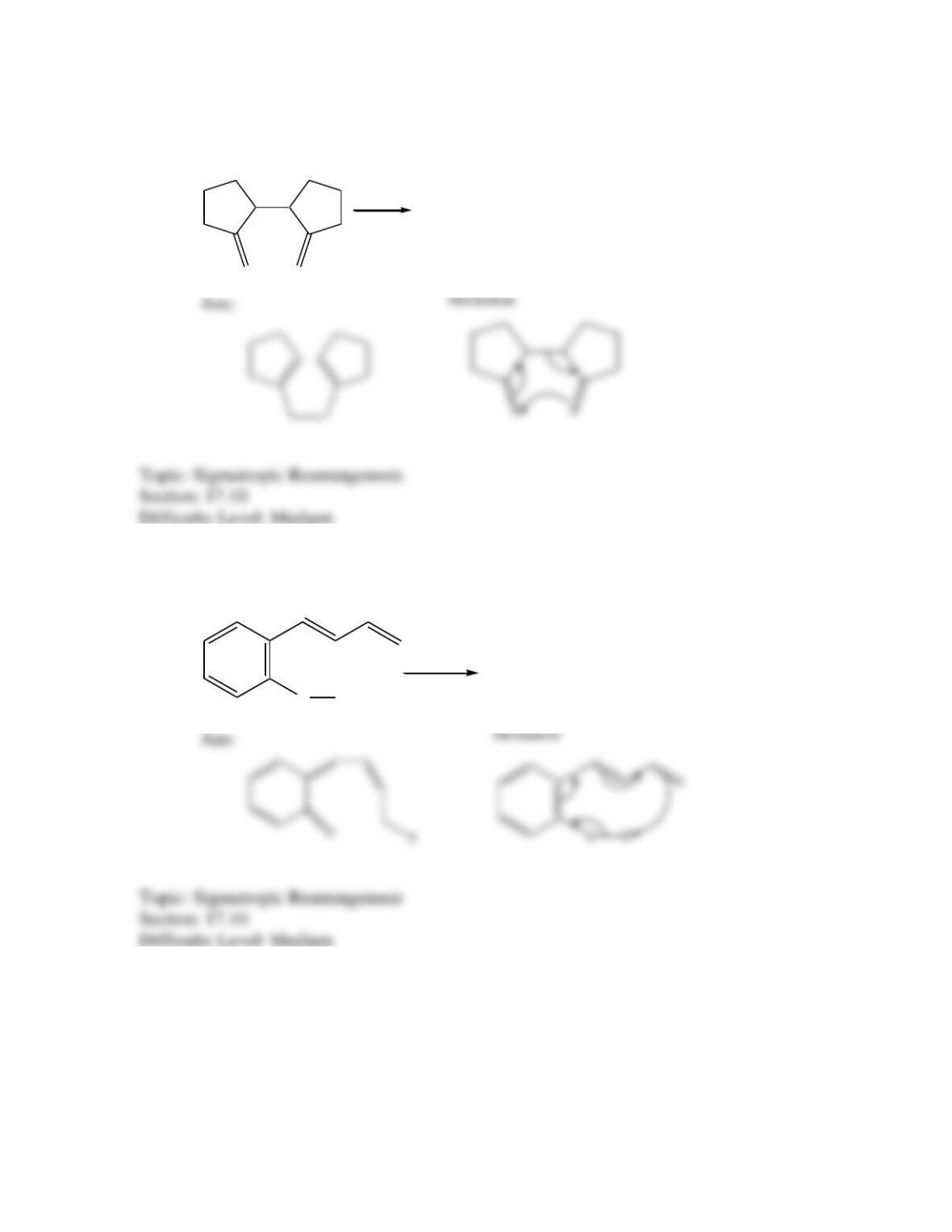
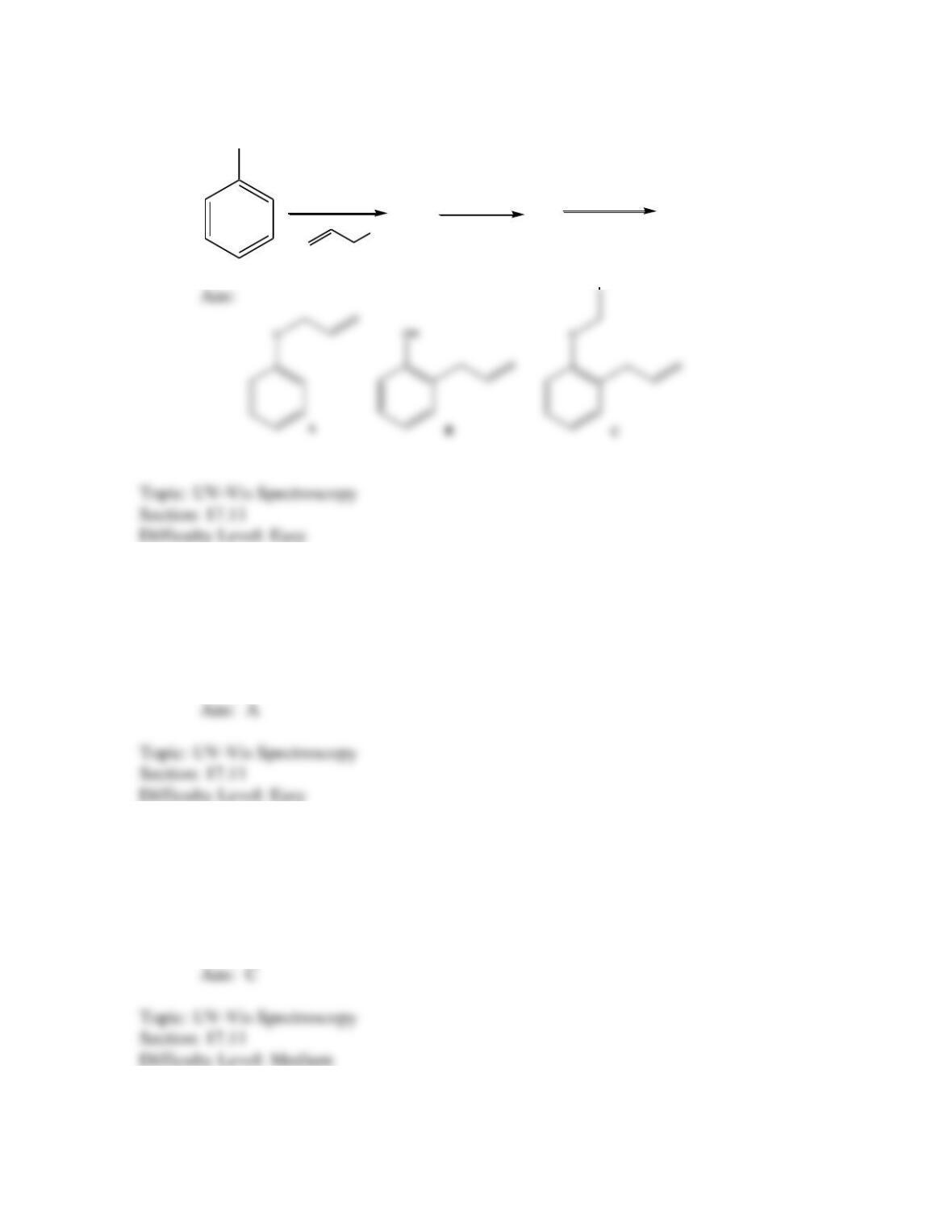
Which of the following best describes the stereochemistry of ring closure and the
product for the following reaction?
disrotatory, cis-3,4-diethylcyclobutene
conrotatory, cis-3,4-diethylcyclobutene
disrotatory, trans-3,4-diethylcyclobutene
conrotatory, trans-3,4-diethylcyclobutene
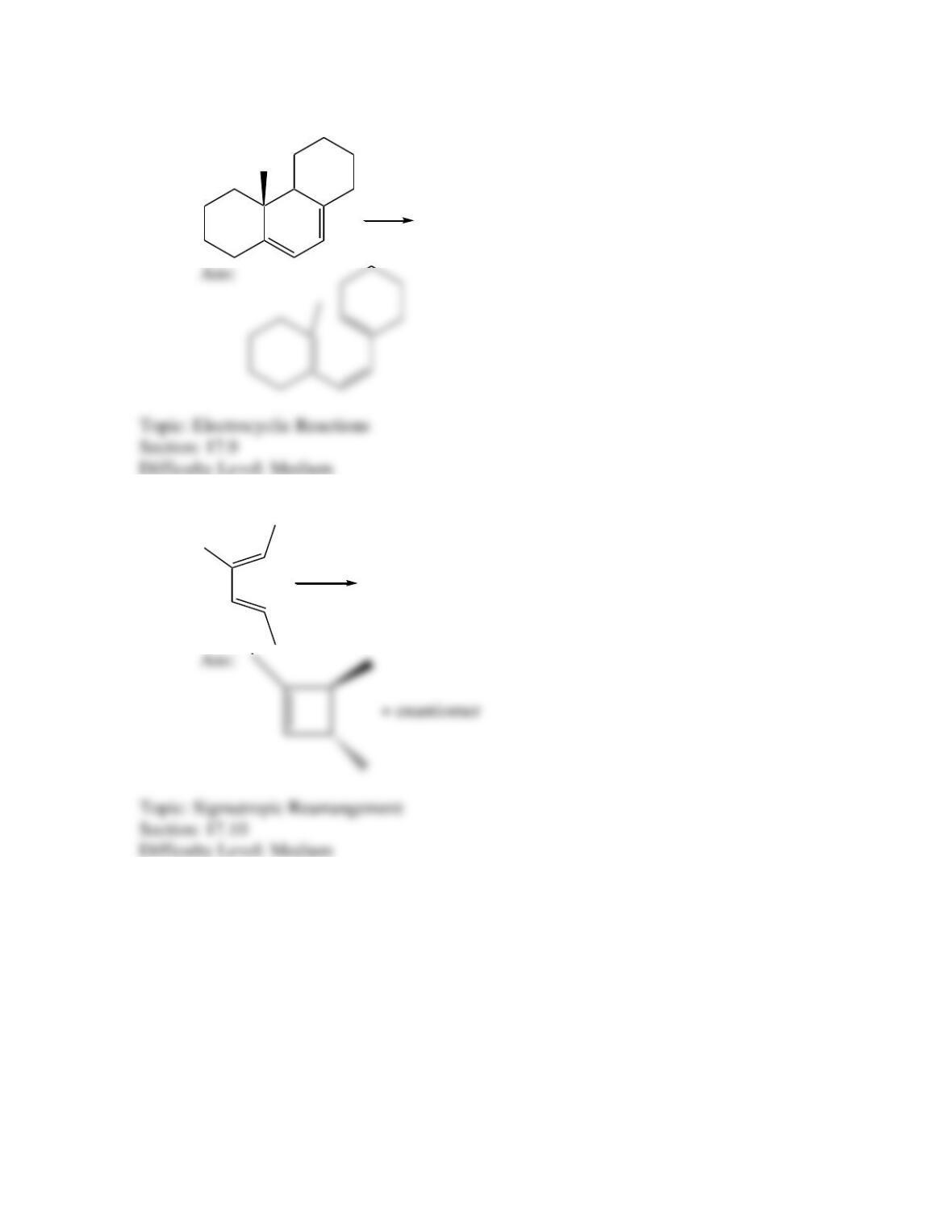
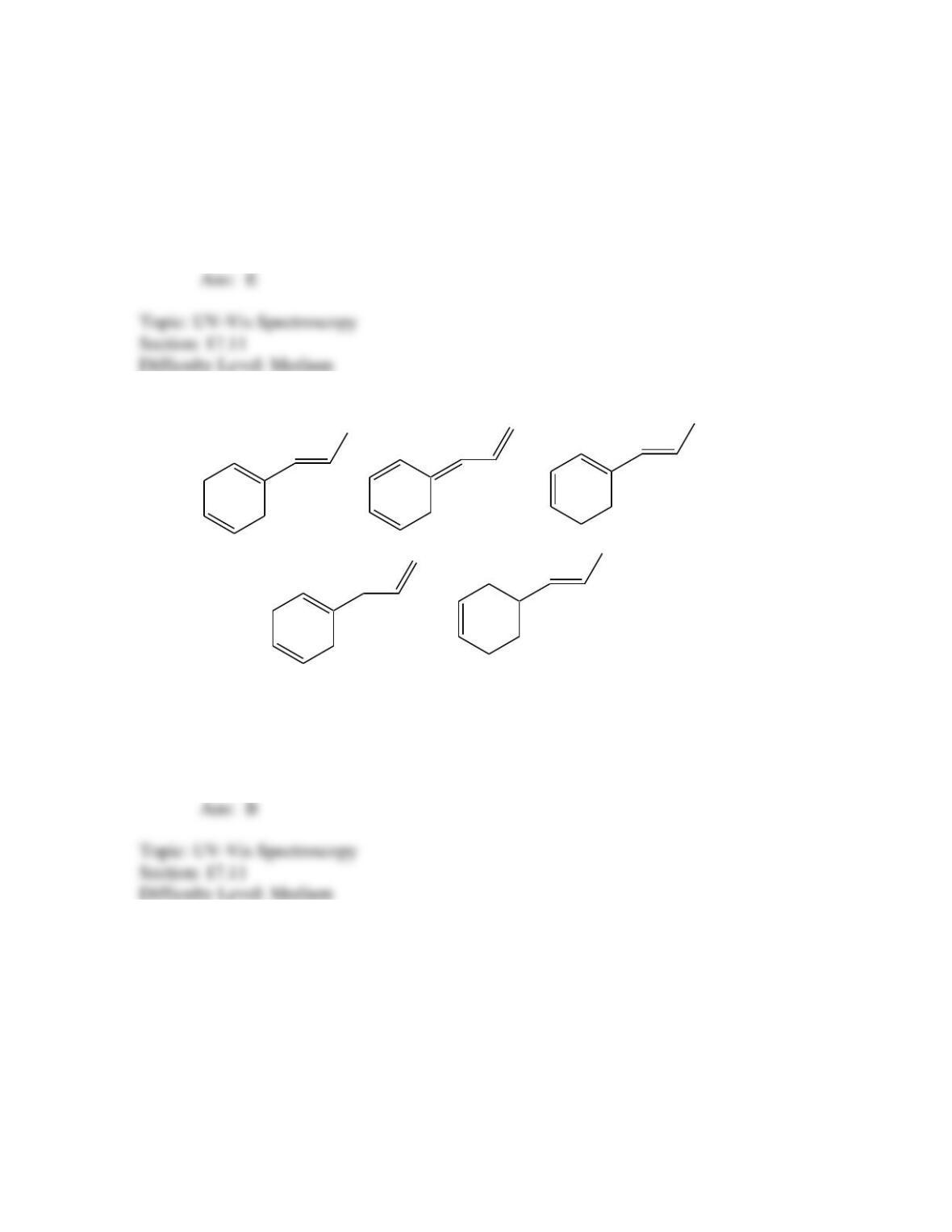
Which of the following best describes the stereochemistry of ring closure and the
product for the following reaction?
disrotatory, cis-3,4-diethylcyclobutene
conrotatory, cis-3,4-diethylcyclobutene
disrotatory, trans-3,4-diethylcyclobutene
conrotatory, trans -3,4-diethylcyclobutene
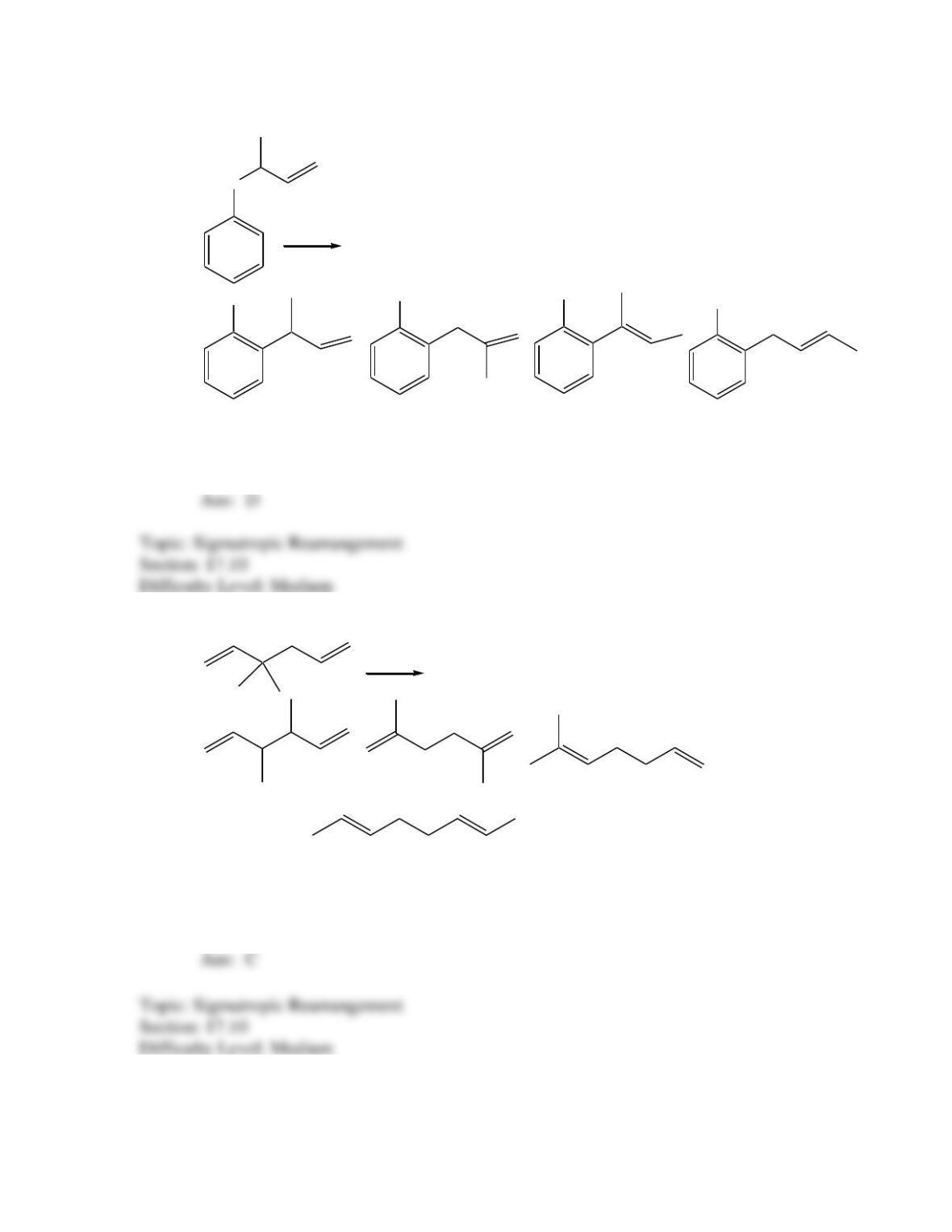
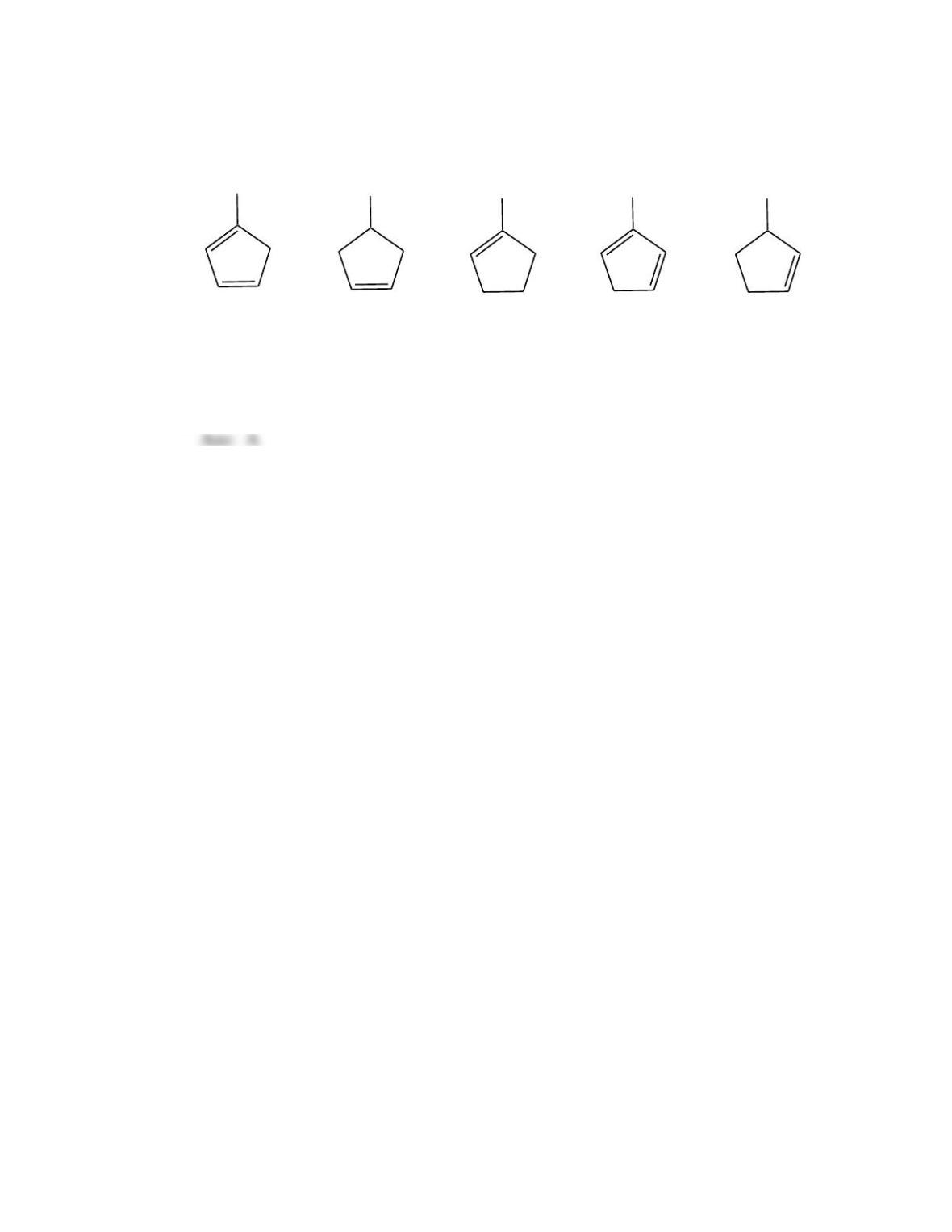
Which of the following best describes the stereochemistry of ring closure and the
product for the following reaction?
h
(3E,5Z,7E)-3,5,7-decatriene
disrotatory, cis-5,6-diethyl-1,3-cyclohexadiene
conrotatory, cis-5,6-diethyl-1,3-cyclohexadiene
disrotatory, trans-5,6-diethyl-1,3-cyclohexadiene
conrotatory, trans -5,6-diethyl-1,3-cyclohexadiene