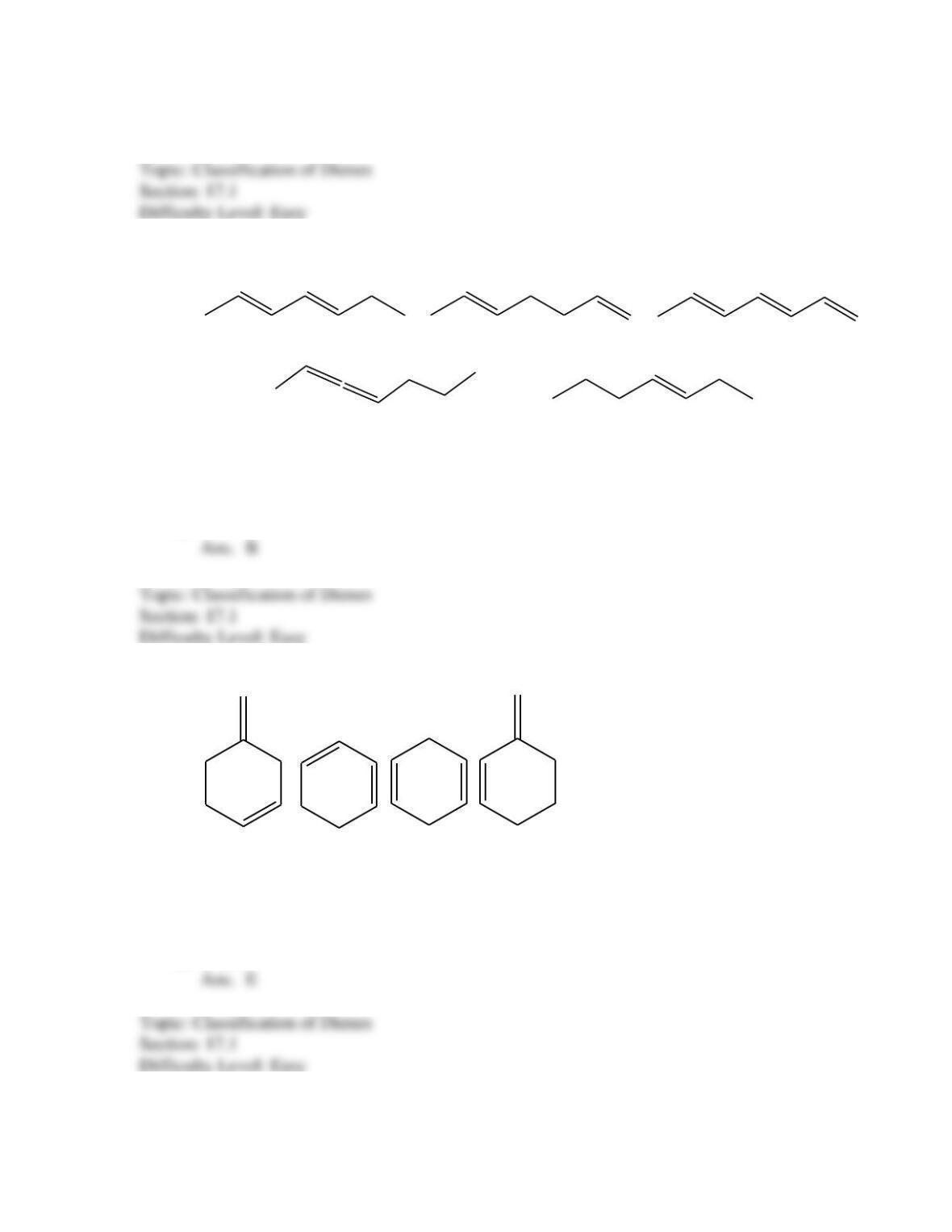
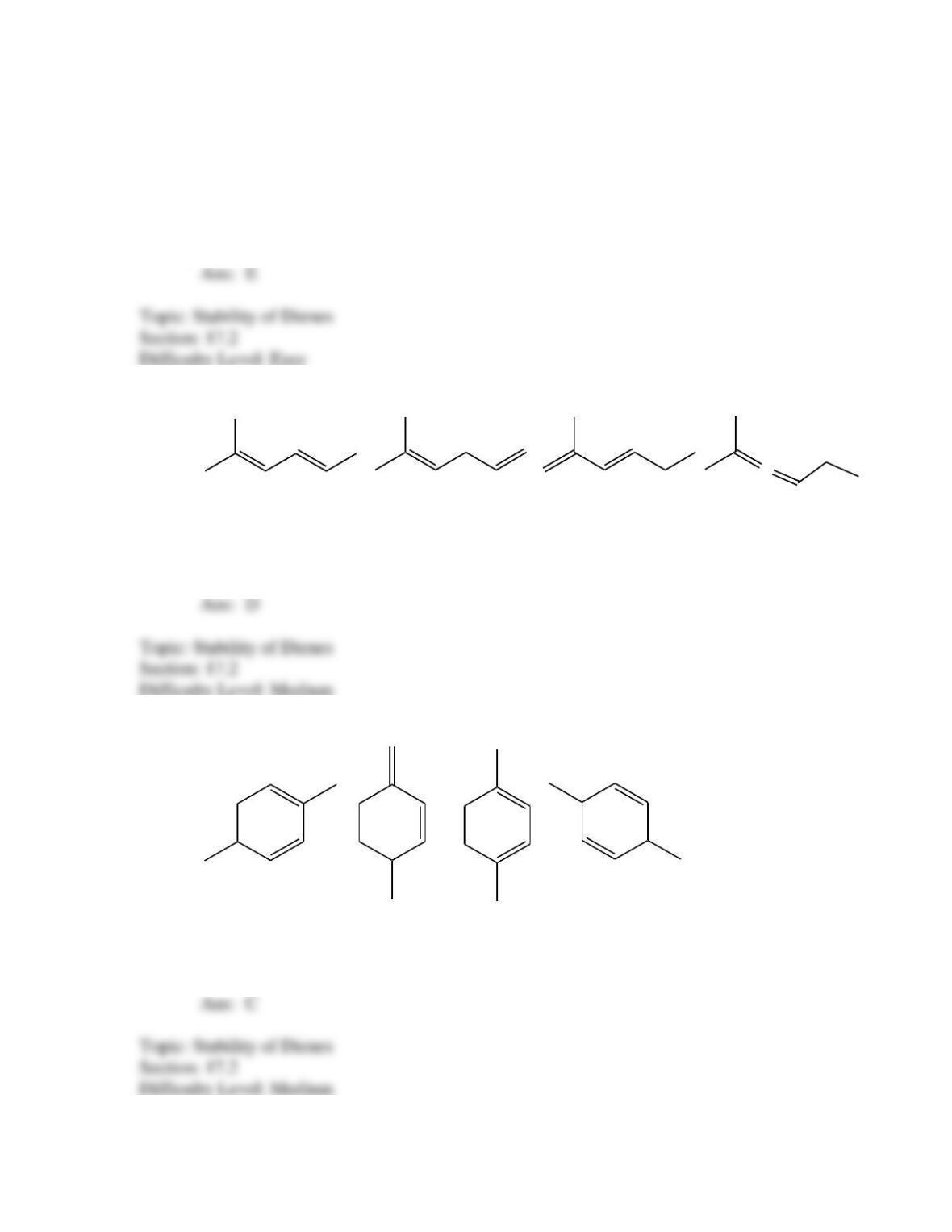
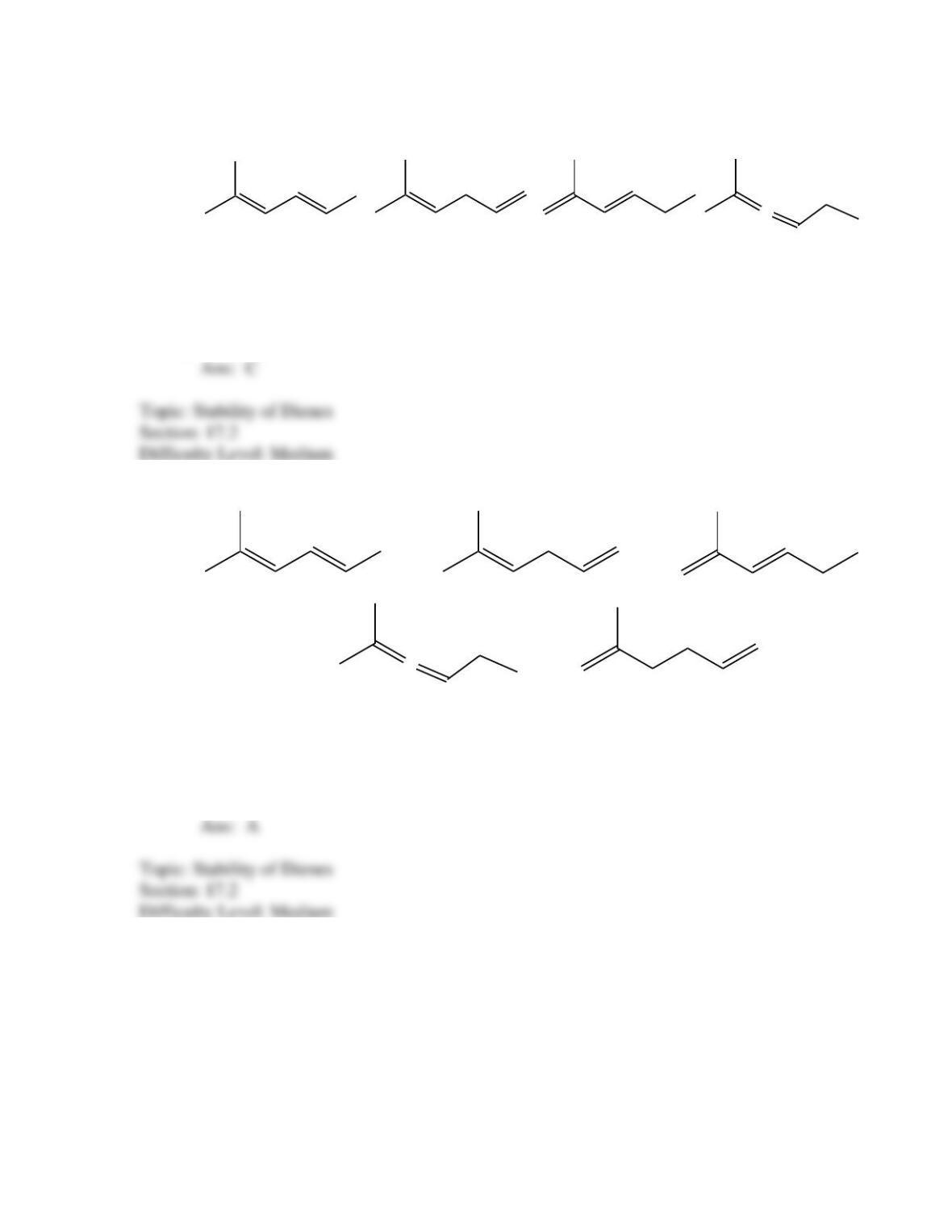
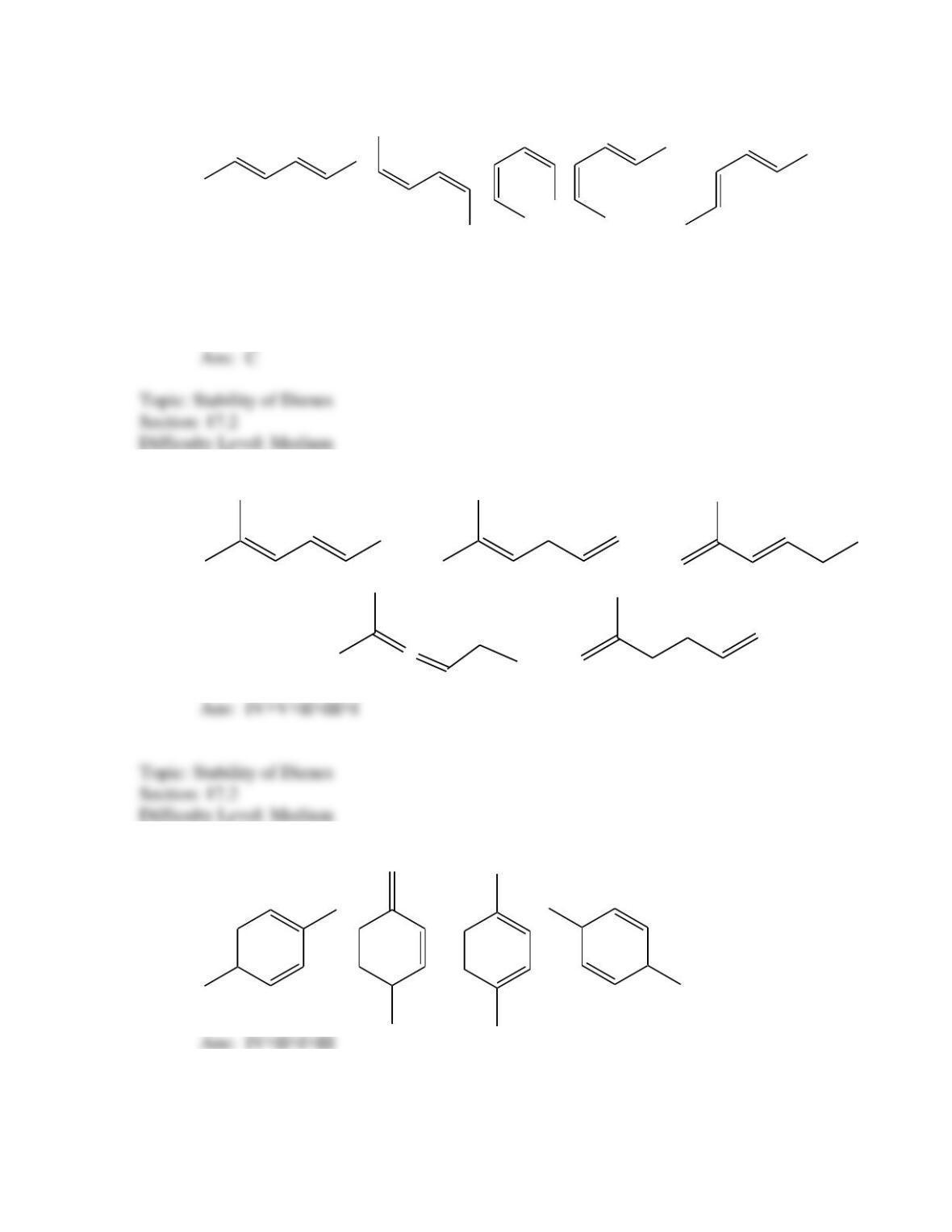
What is the IUPAC name for the following compound?
1-chloro-1-methyl-2,5-cyclohexadiene
3-chloro-3-methyl-1,4-cyclohexadiene
6- chloro-6-methyl-1,4-cyclohexadiene
2- chloro-2-methyl-1,3-cyclohexadiene
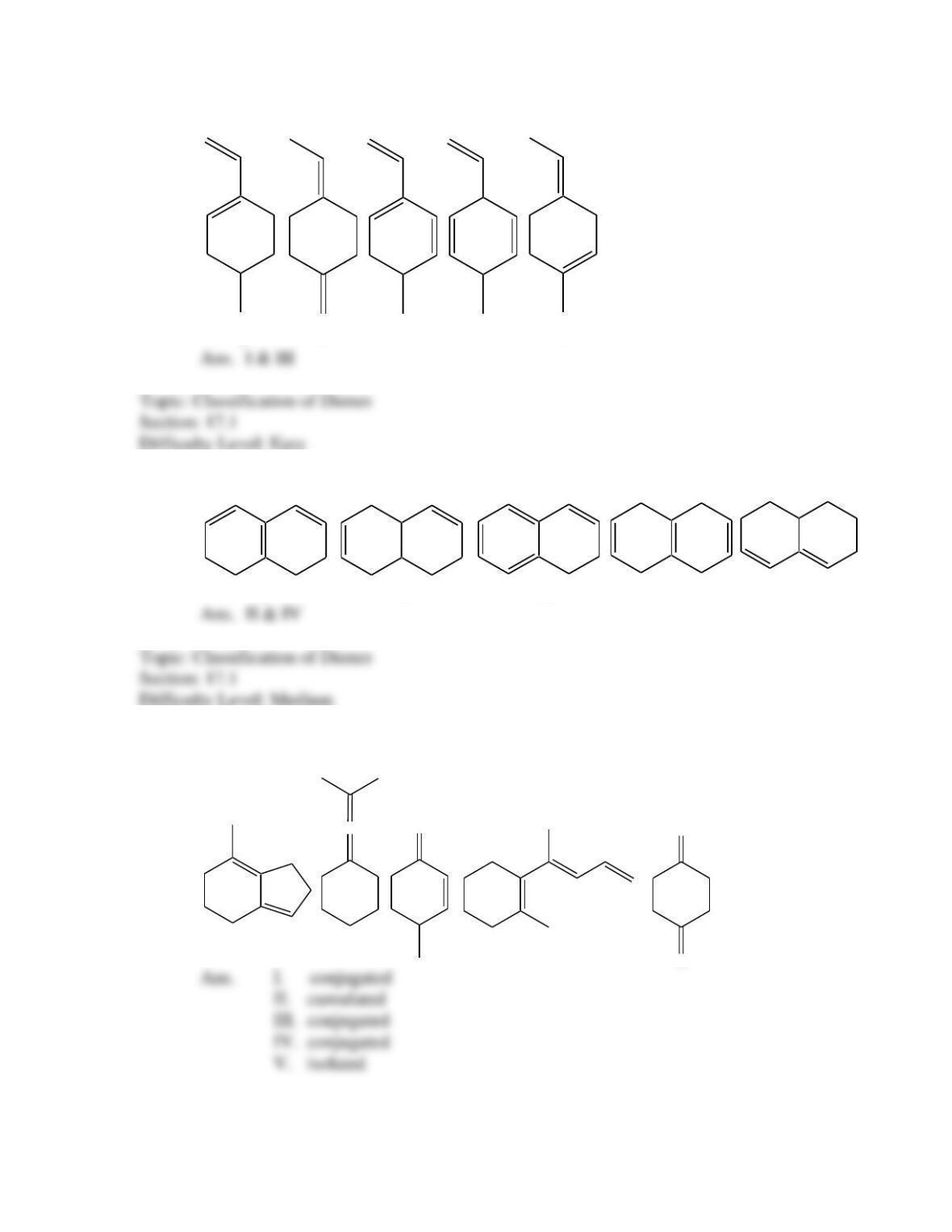
What is the IUPAC name for the following compound?
(2E, 4Z)-1,4-dimethyl-1,3-butadiene
(2Z, 4Z)-1,4-dimethyl-1,3-butadiene
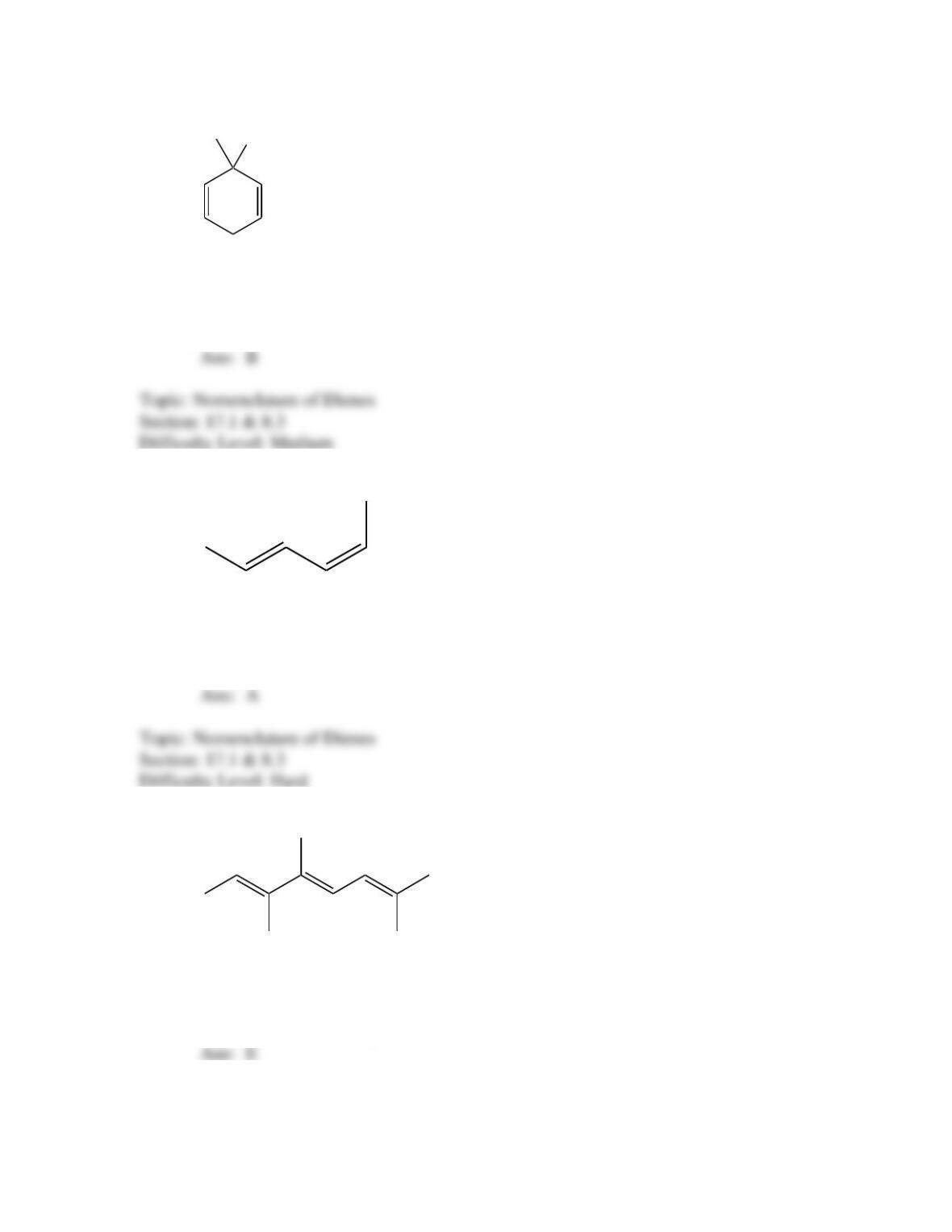
What is the IUPAC name for the following compound?
(2E,4Z,6E)–3,4,7,8–tetramethyl-2,4,6–heptatriene
(2Z,4E,)–3,4,7–trimethyl-2,4,6–octatriene
(2E,4Z,6E)–2,5,6,7–tetramethyl-3,5,7–heptatriene
(2E,4Z)– 2,5,6–trimethyl-3,5,7–octatriene
(2E,4E,)–2,5,6–trimethyl-2,4,6–octatriene