78. To add one carbon directly to the end of a primary alkyl bromide, one could:
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene.
79. To remove one carbon from the end of a primary alkyl bromide, one could:
A) substitute bromide with acetylide, then cleave the triple bond.
B) substitute bromide with acetylide, then reduce the alkyne to an alkene.
C) substitute bromide with methoxide.
D) eliminate hydrogen bromide to produce an alkene, then cleave the
double bond.
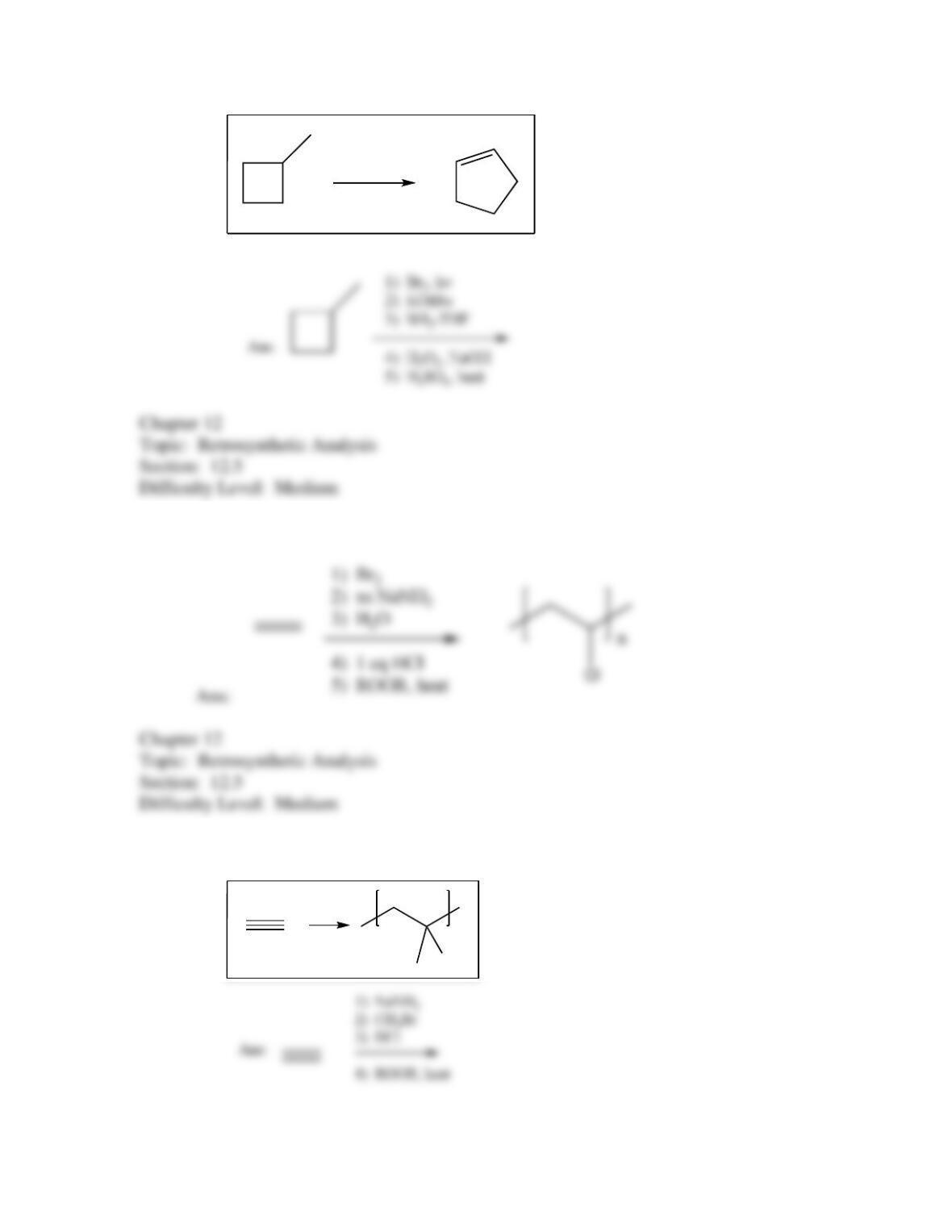
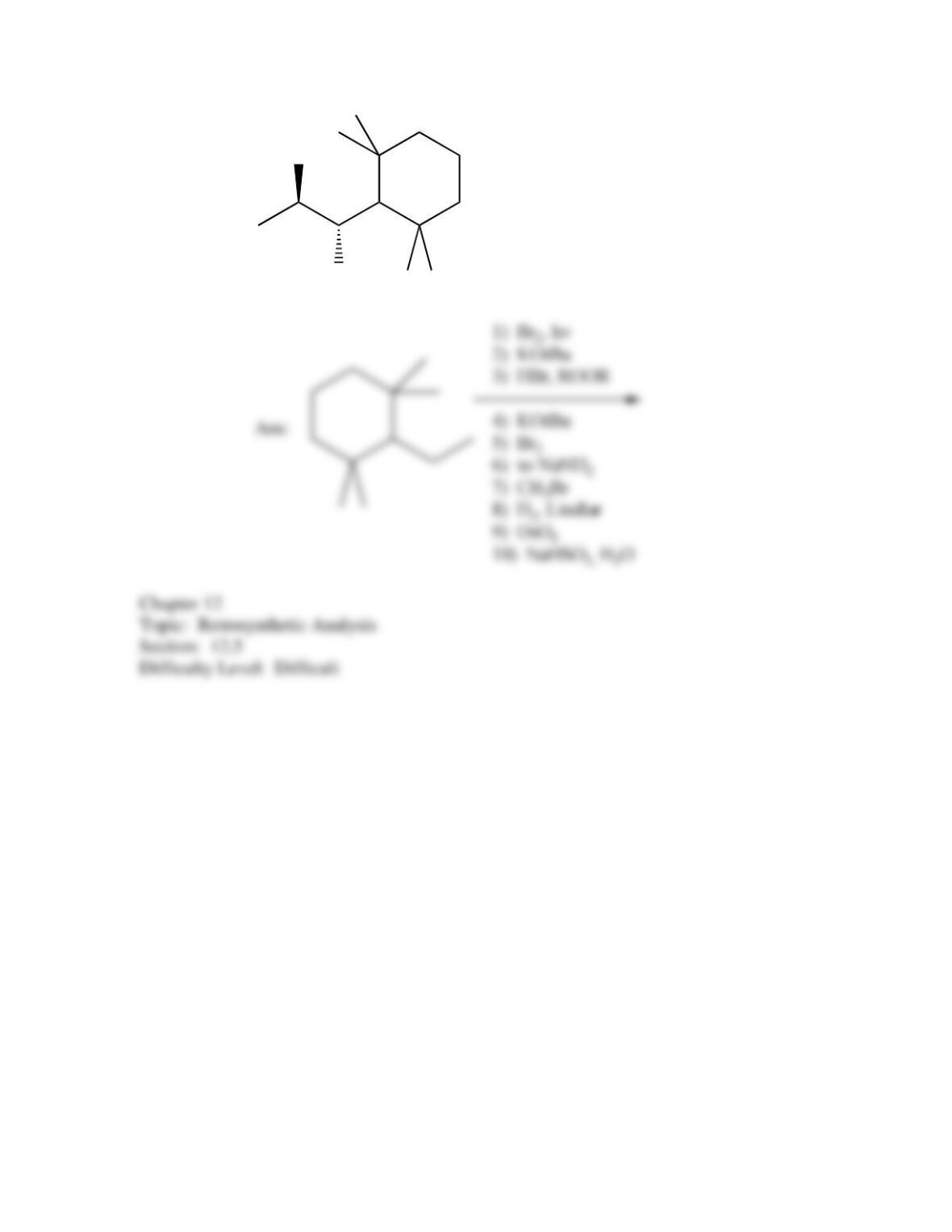
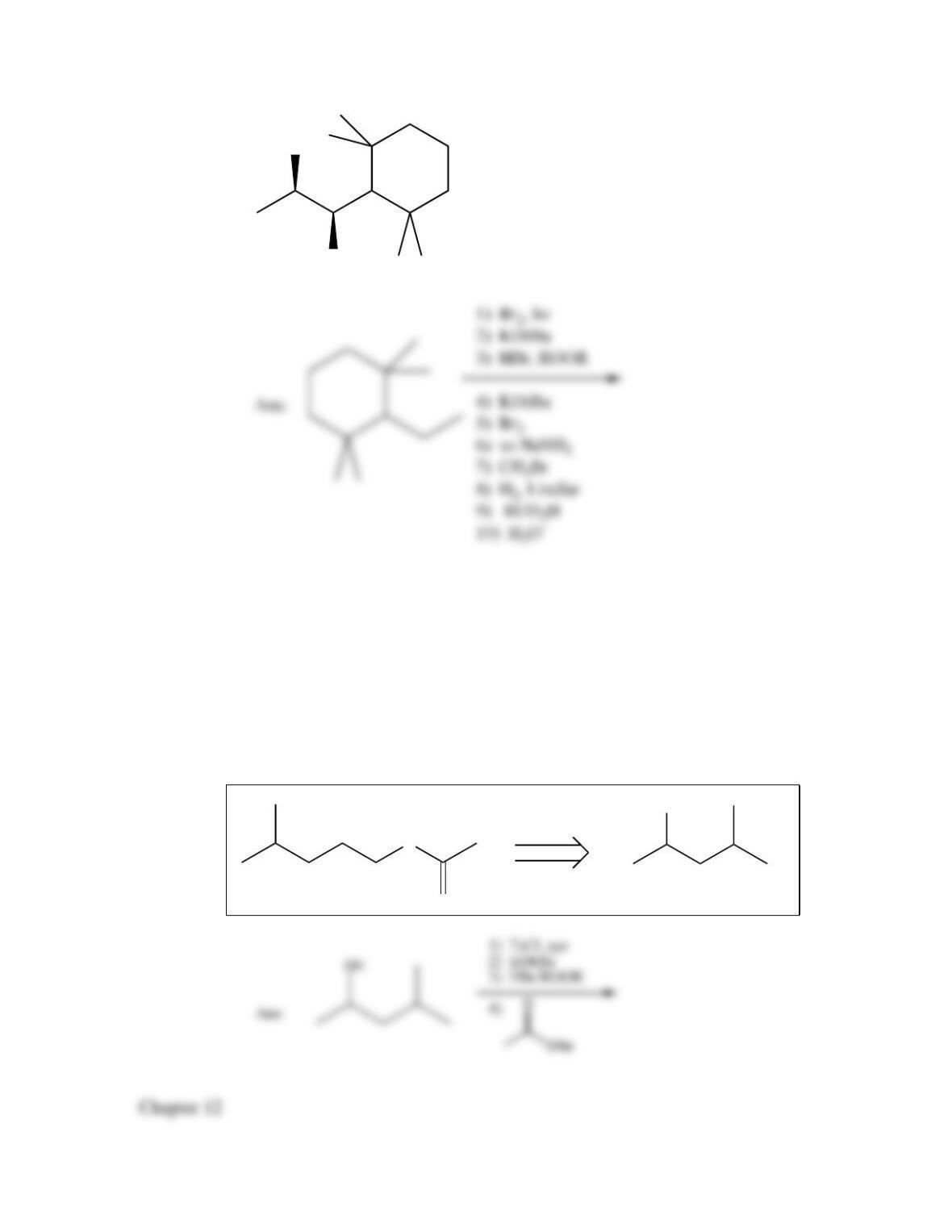
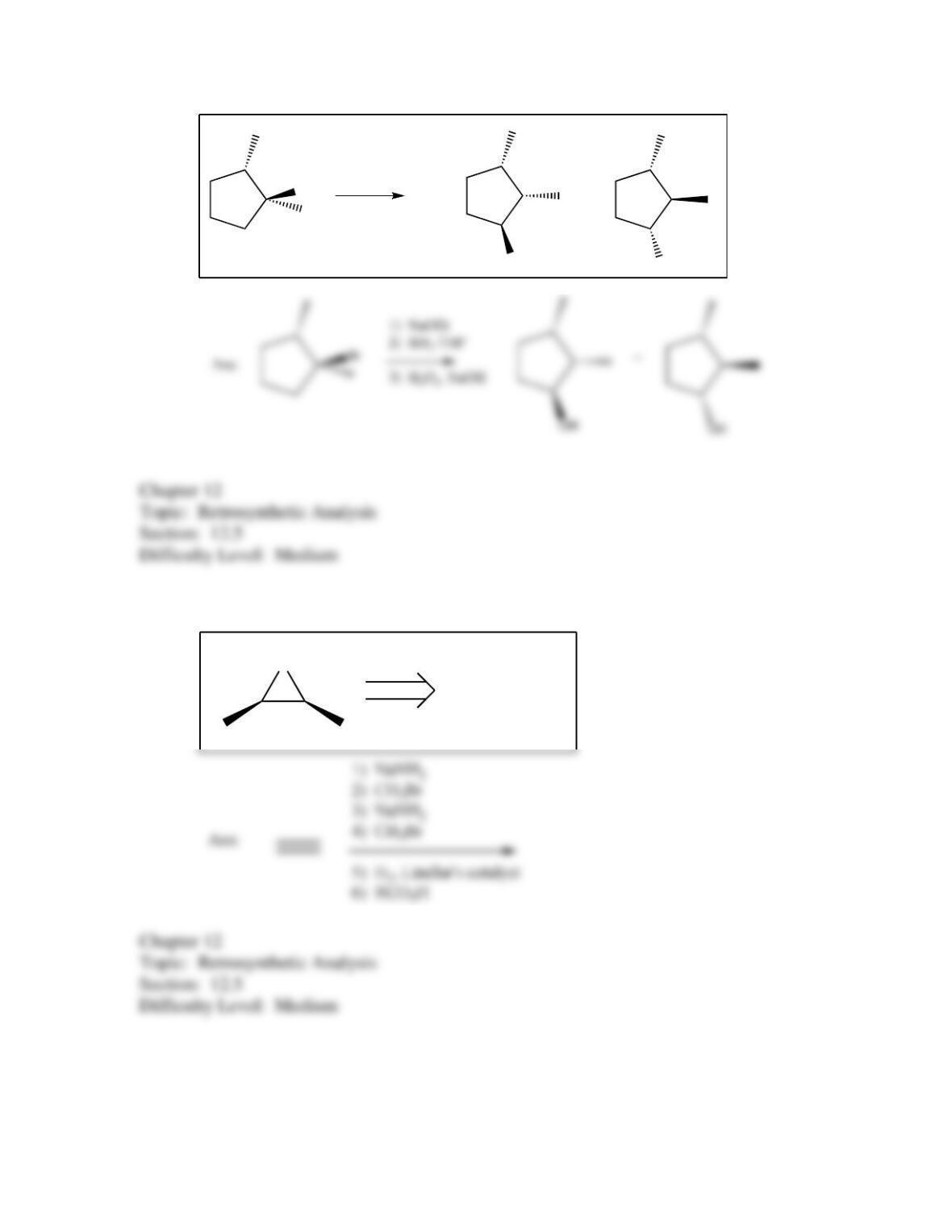
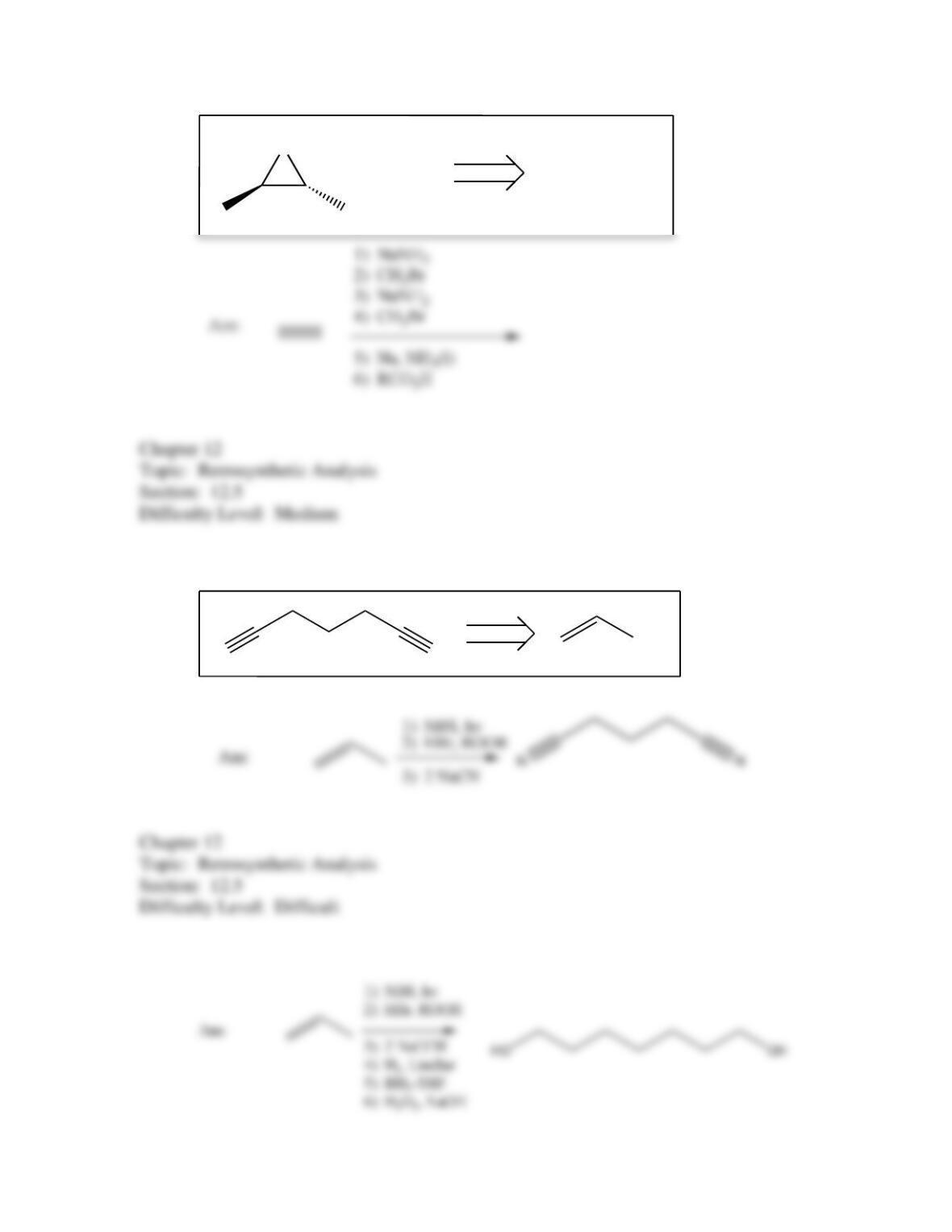
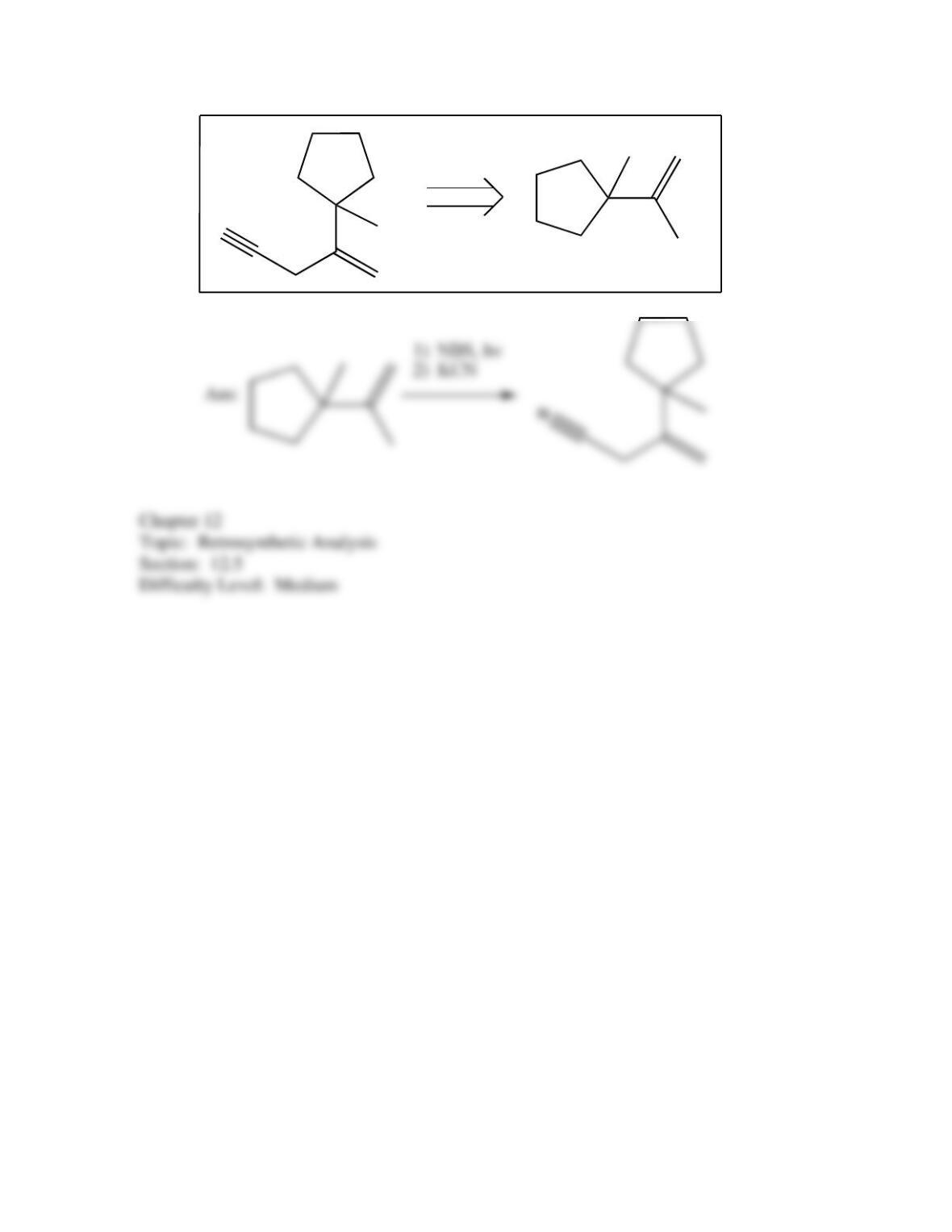
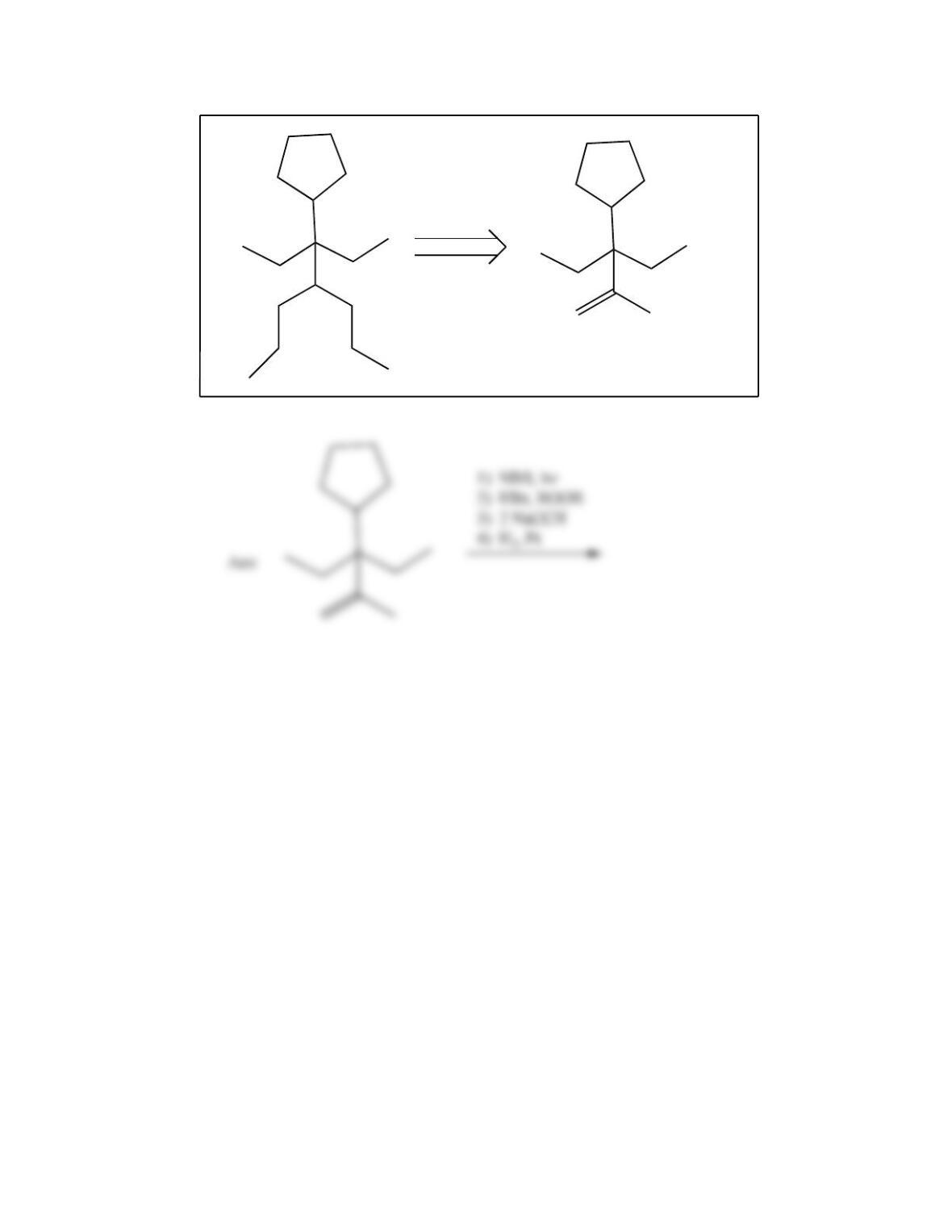
80. Devise a method to complete the following synthesis.