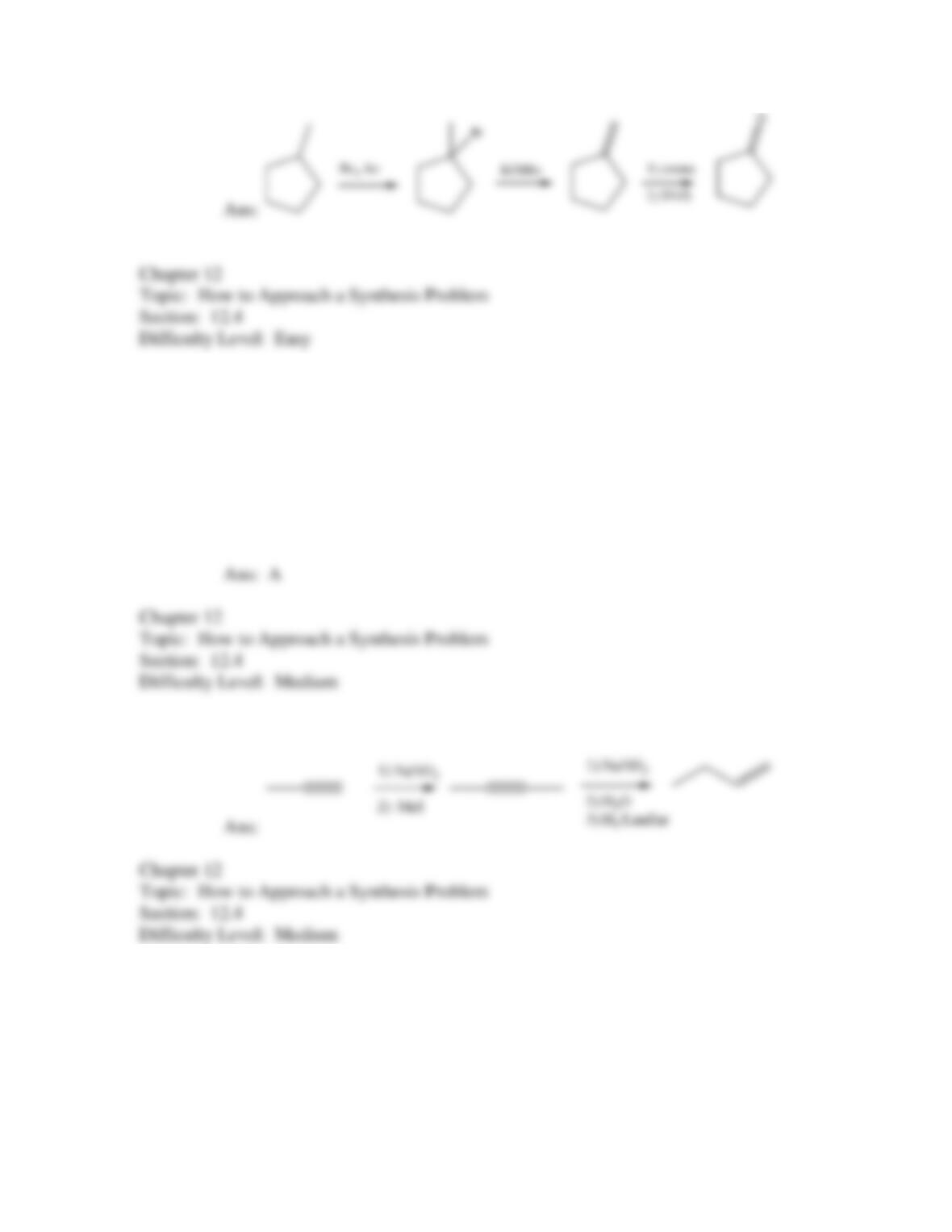
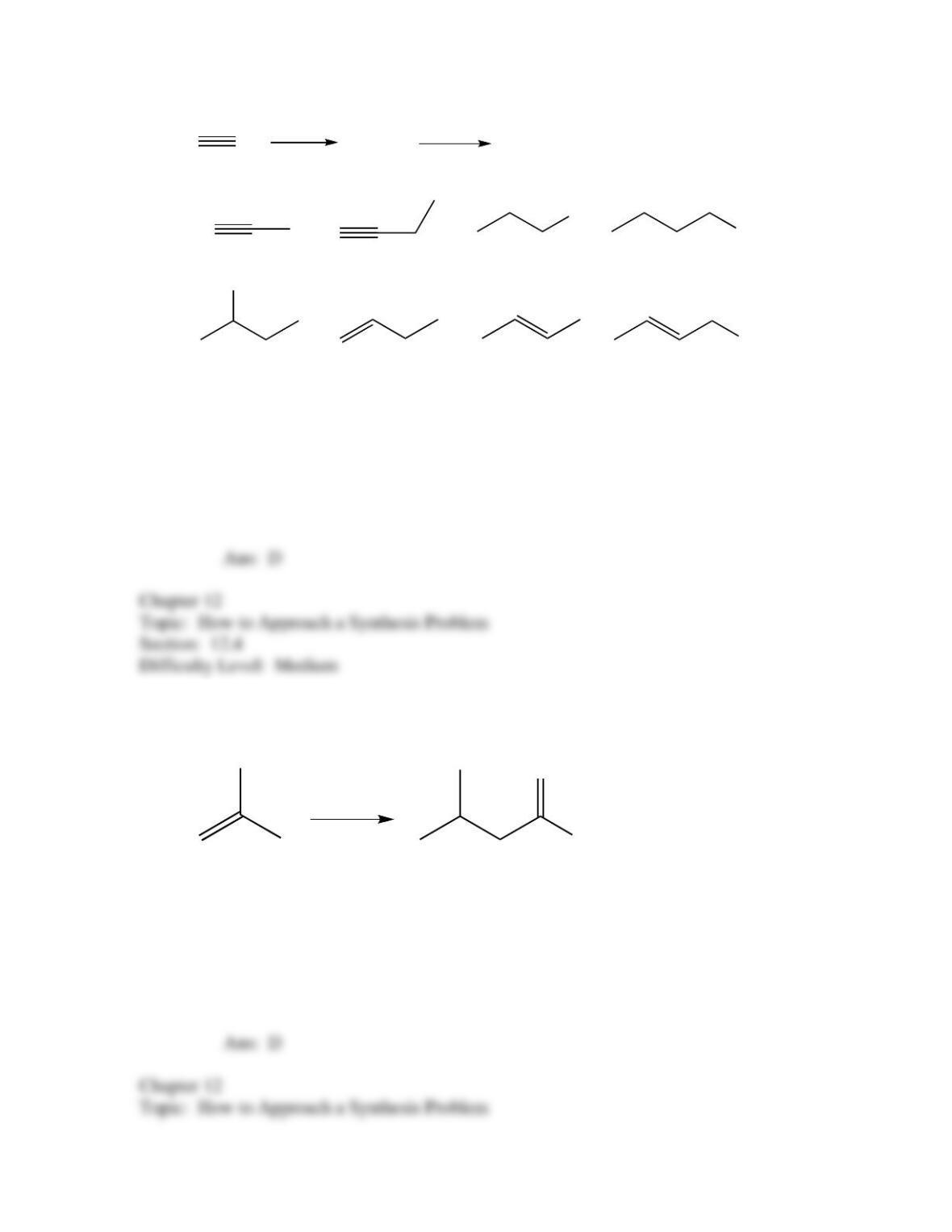
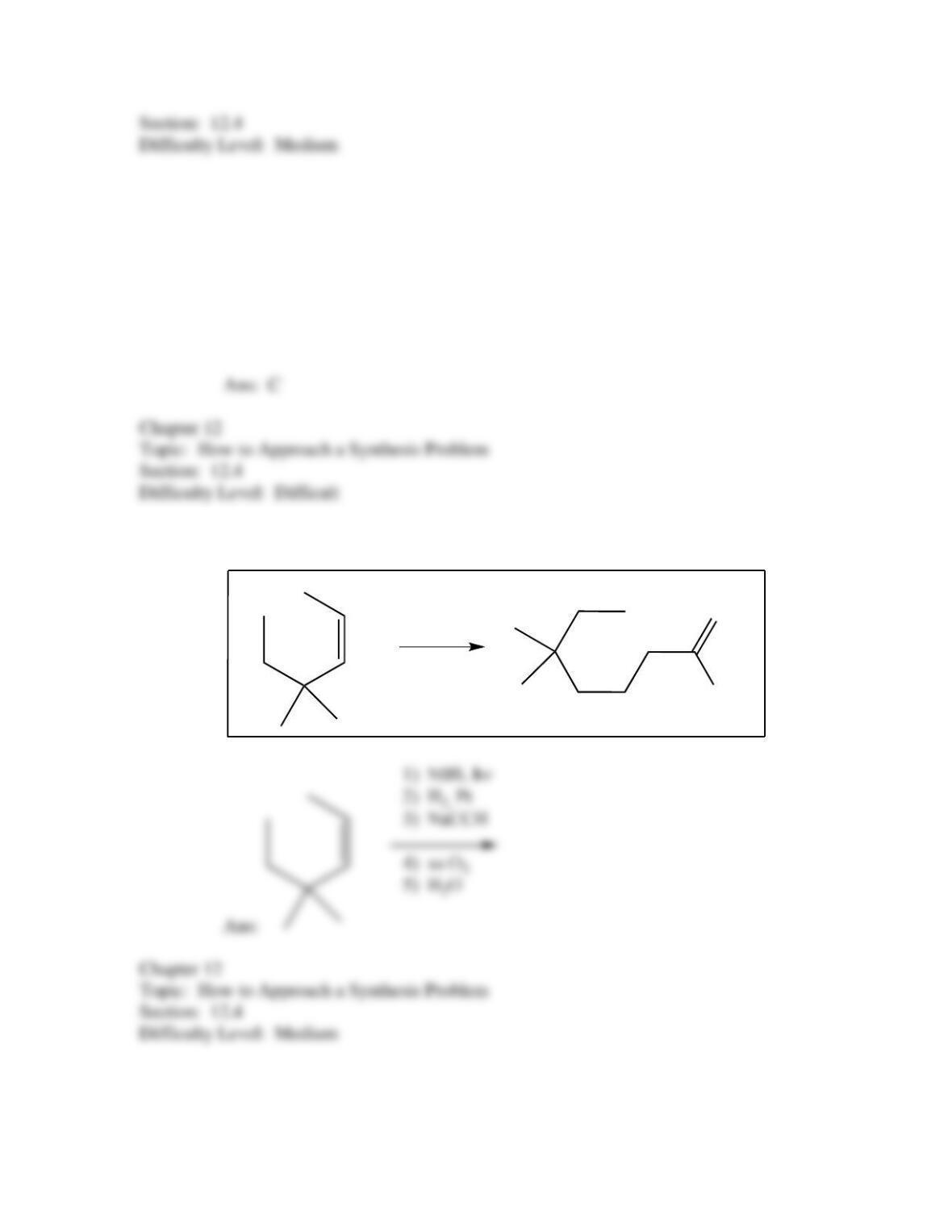
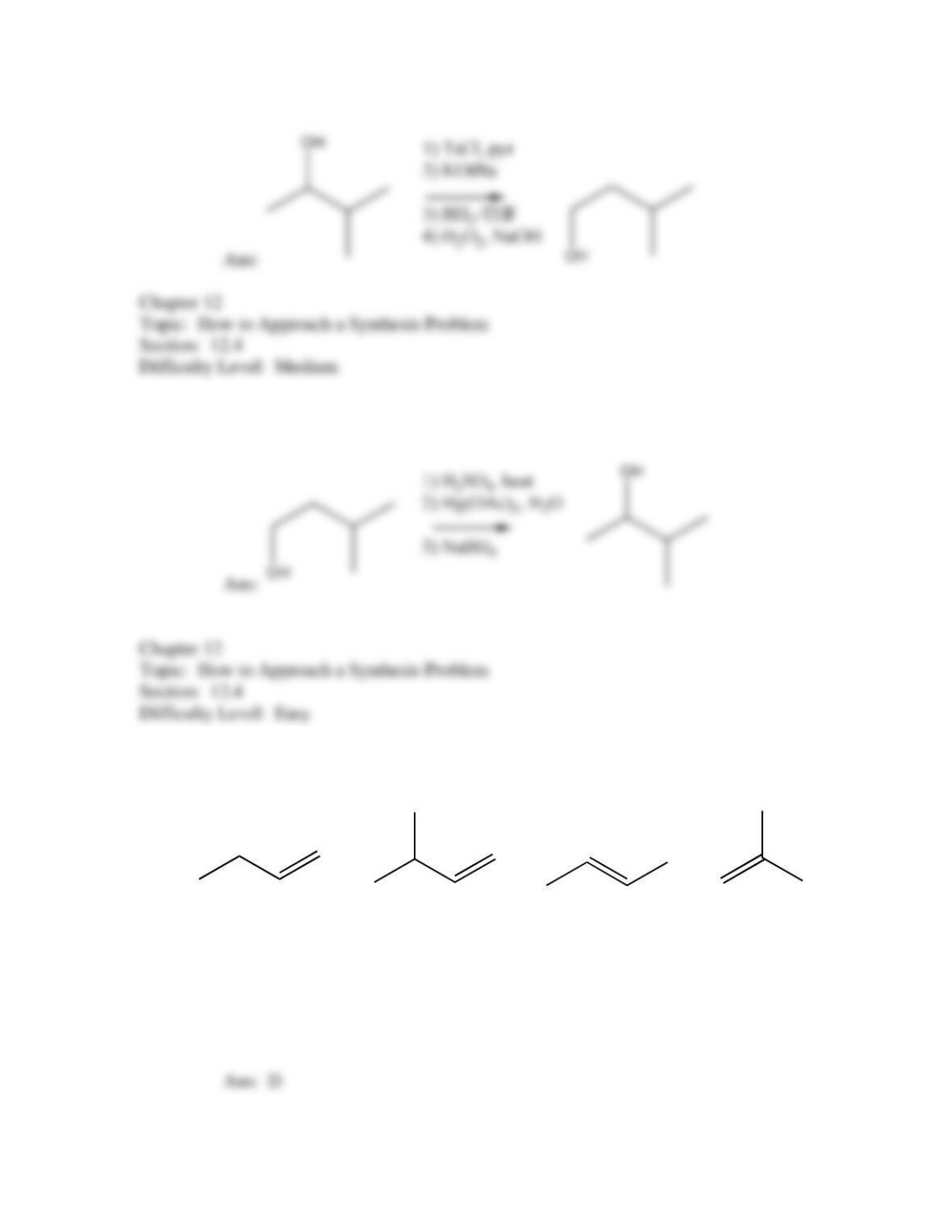
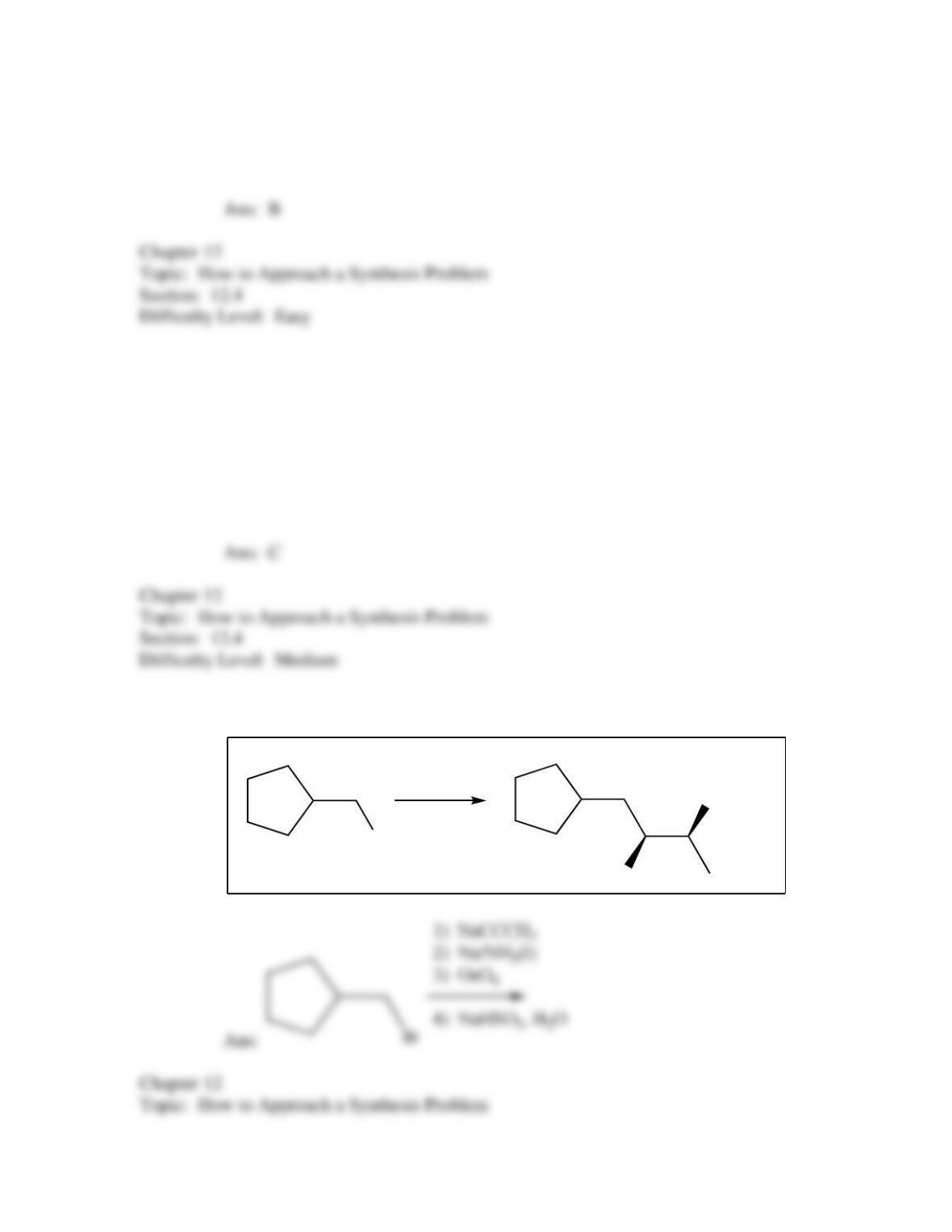
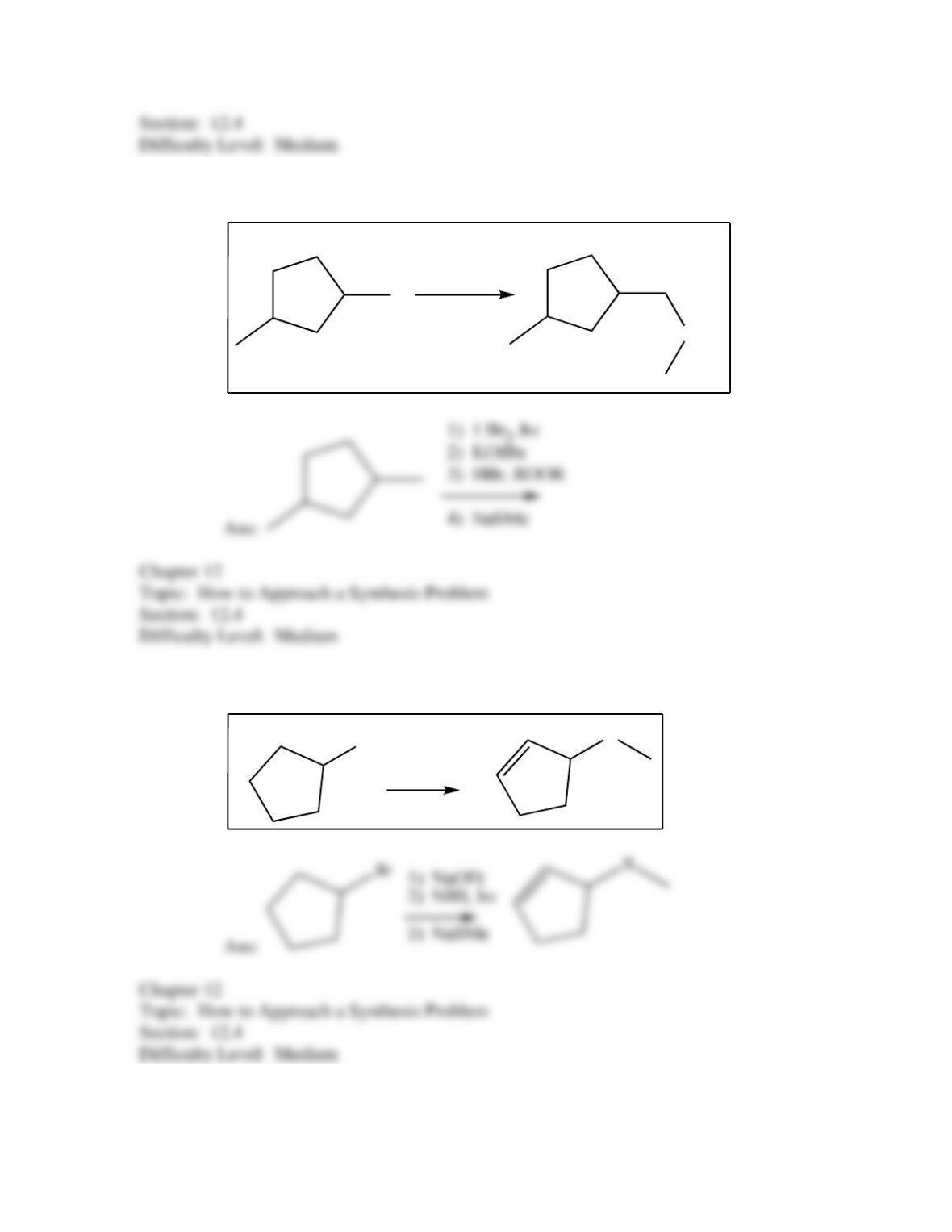
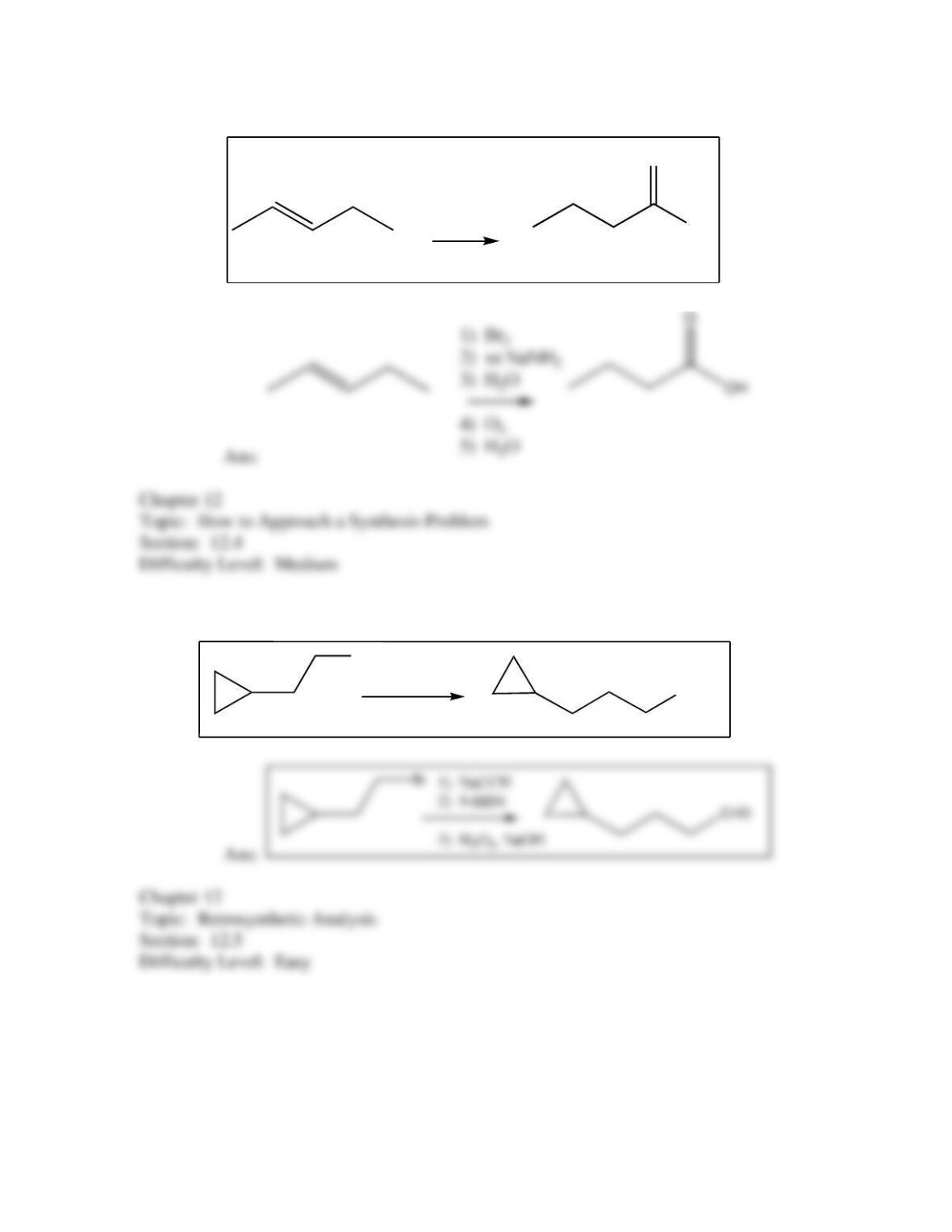
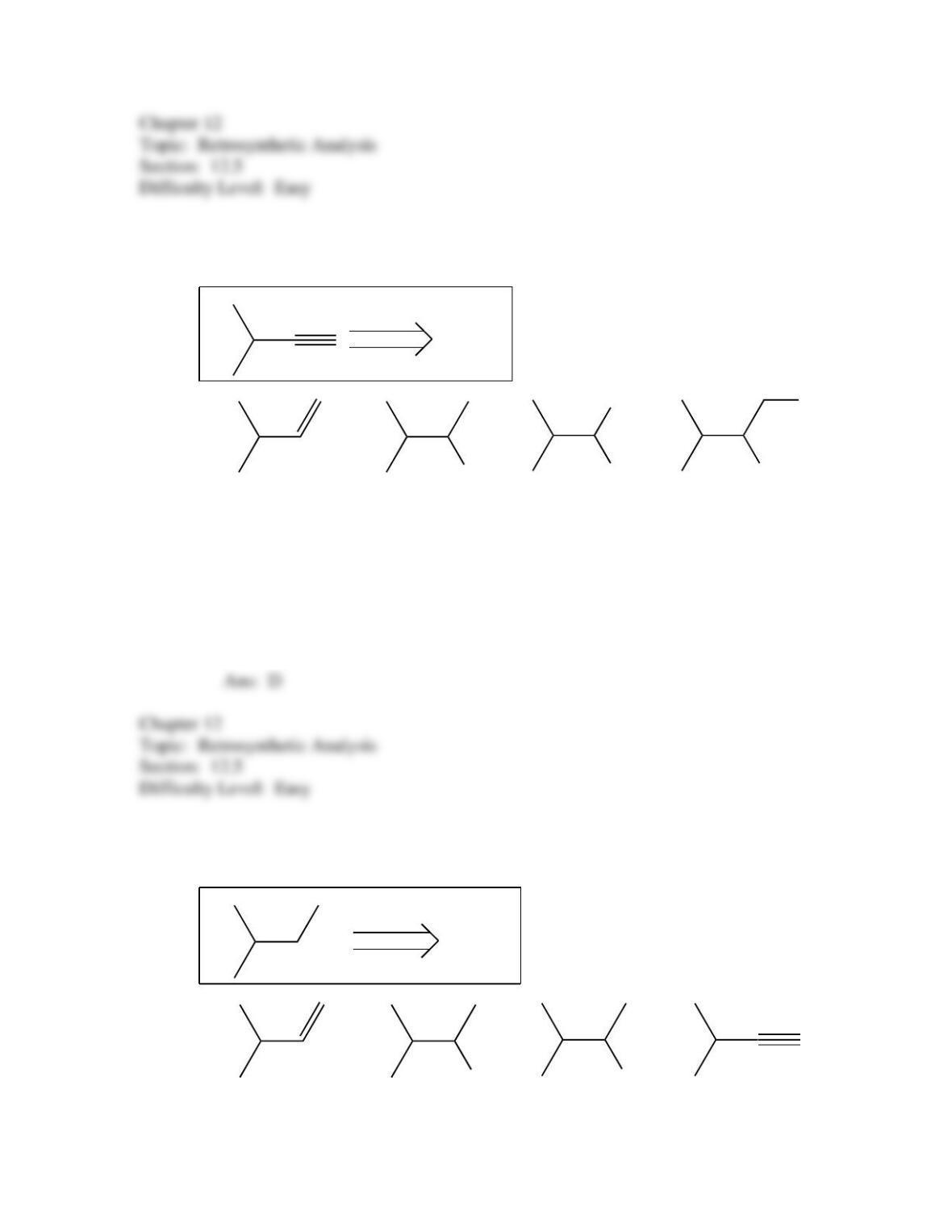
50. Which of the following provides an efficient method of converting 3-bromo-2-
methyl-1-butene into 2-methyl-2-butene?
A) 1) NaOH; 2) H2, Pt
B) 1) H2, Pt; 2) NaOEt
C) 1) H2, Pt; 2) xs NaNH2
D) 1) H2, Ni2B; 2) KOtBu
E) 1) H2, Pt; 2) KOtBu
51. Which order of alterations would most effectively transform trans-2-butene into
1-butene?
A) convert to the dibromoalkane, then to the terminal alkyne, then to the
terminal alkene
B) convert to an alcohol, then to a terminal alkyne, finally to the terminal
alkene
C) convert to the terminal alkene in one step
D) shorten the chain by two carbons, then add a two-carbon alkene to the
end
E) convert to an alkane, then to a terminal alkyne, and finally to a terminal
alkene
52. What is the minimum number of steps required to convert 2-methylpropane into
2-methylpropene?
A) 1
B) 2