C) Hept-3-en-6-yne
D) Hept-4-en-1-yne
32. Reaction of compound A (molecular formula = C7H12) with sodium amide
followed by reaction with 1-bromobutane produces
(CH3)2CHCH(CH3)CC(CH2)3CH3. What is the name of compound A?
A) 2,3-dimethylnon-4-yne
B) 2,2-dimethylpent-1-yne
C) 3,4-dimethylpent-1-yne
D) 4,4-dimethylhept-1-yne
E) 1-heptyne
33. Reaction of compound A (molecular formula = C12H22) with ozone followed by
reaction with DMS produces only (CH3)3CCH2CHO. Which of the following
could be compound A?
A) cis-2,3,7,8-tetramethyloct-4-ene
B) trans-dodec-6-ene
C) 4,4-dimethylpent-1-ene
D) cis-2,2,7,7-tetramethyloct-4-ene
E) 2,2-dimethyldec-4-ene
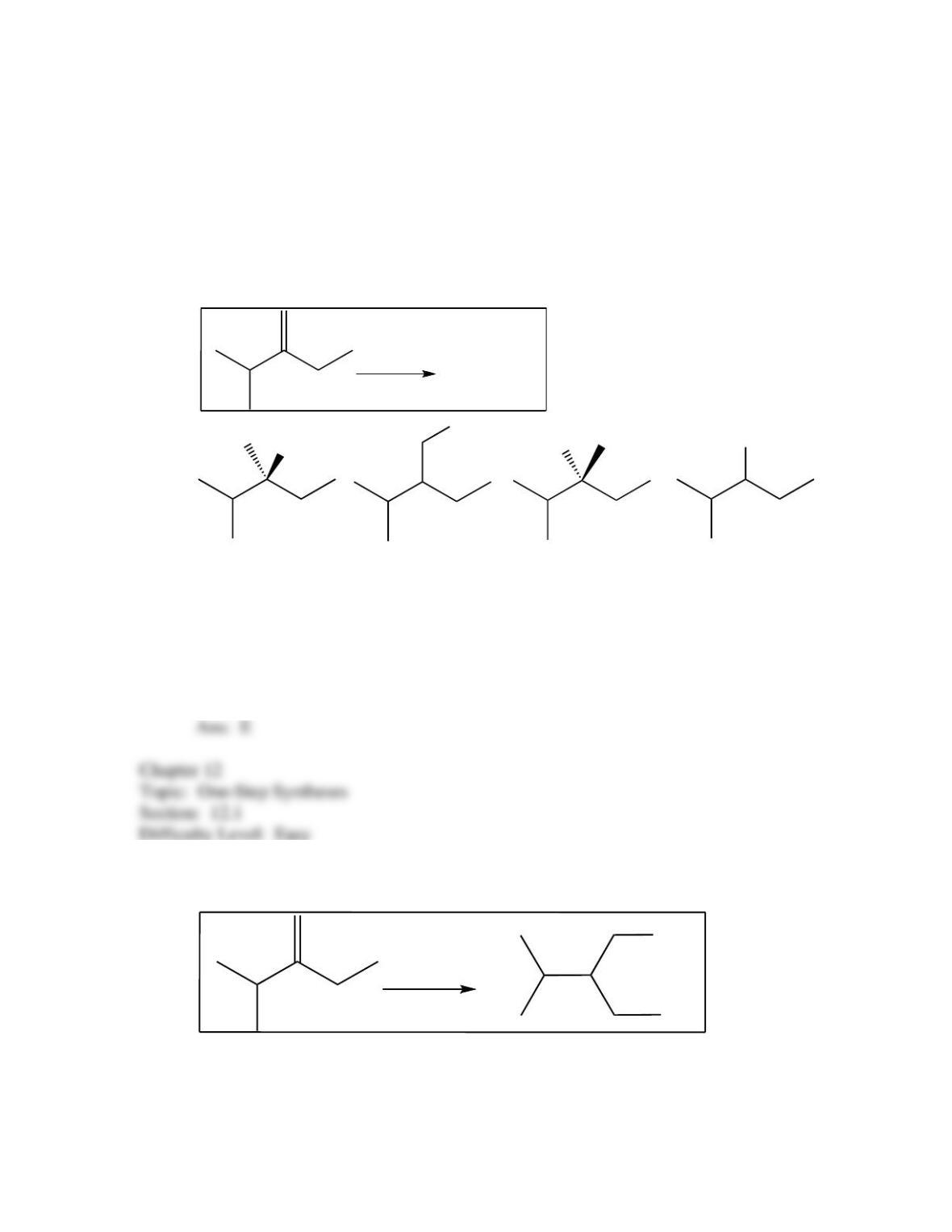
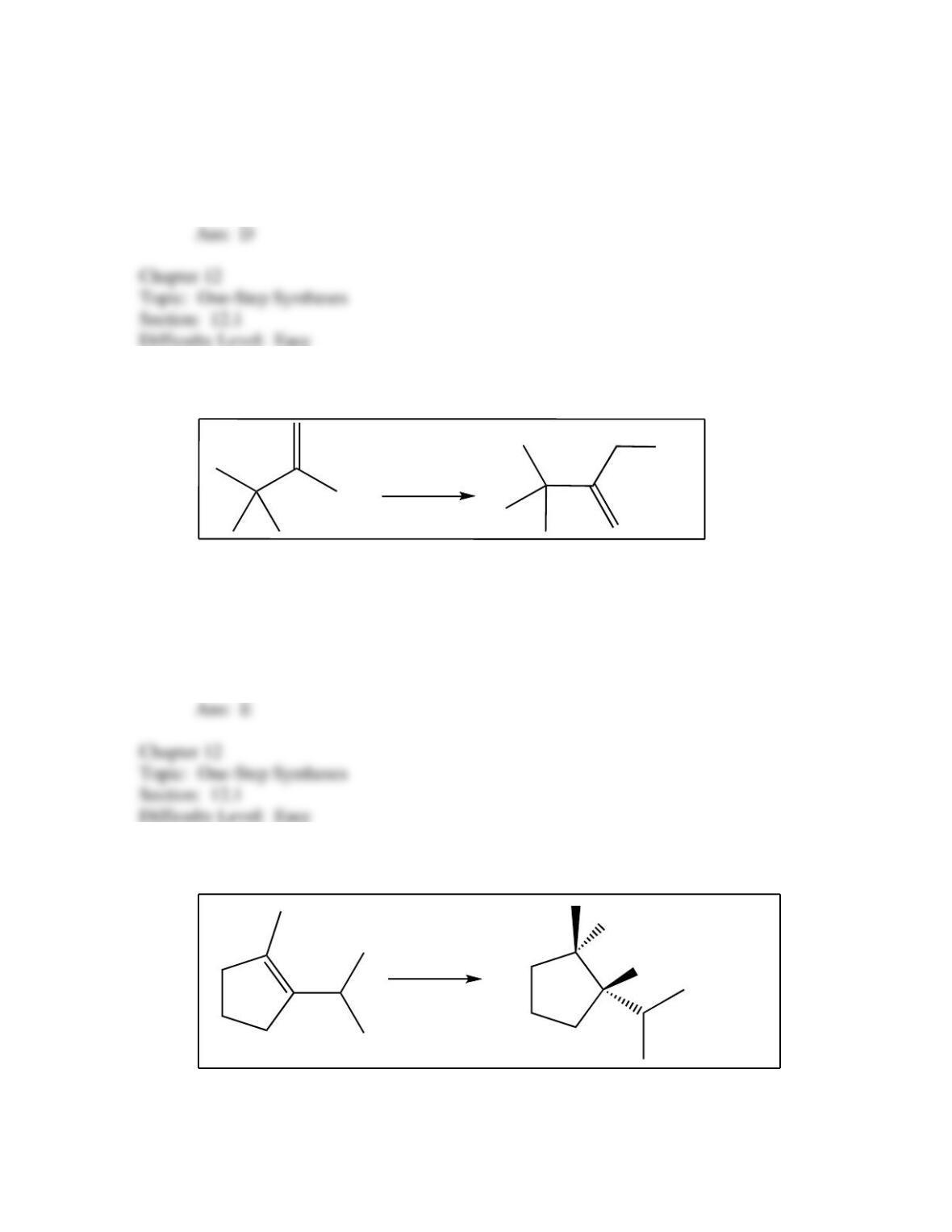
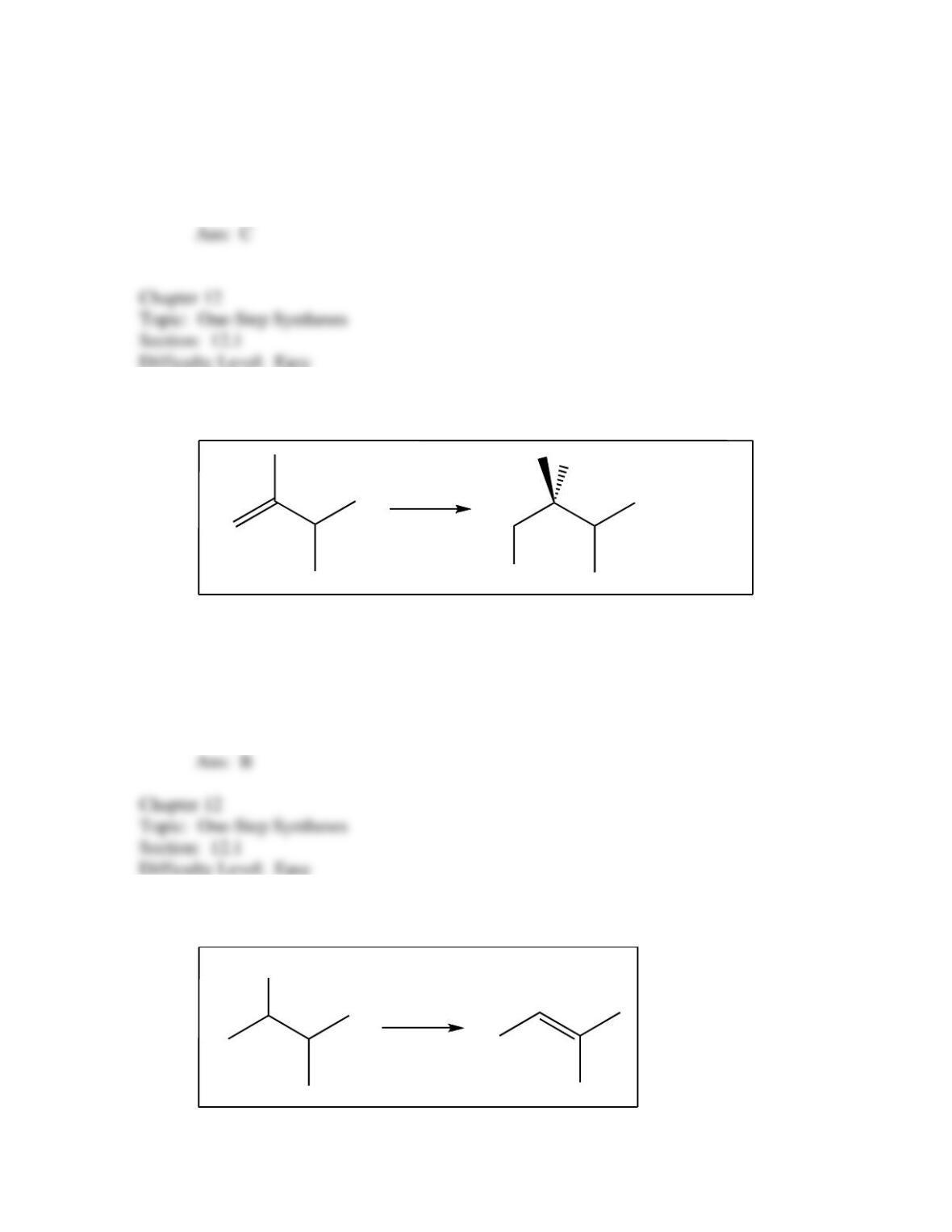
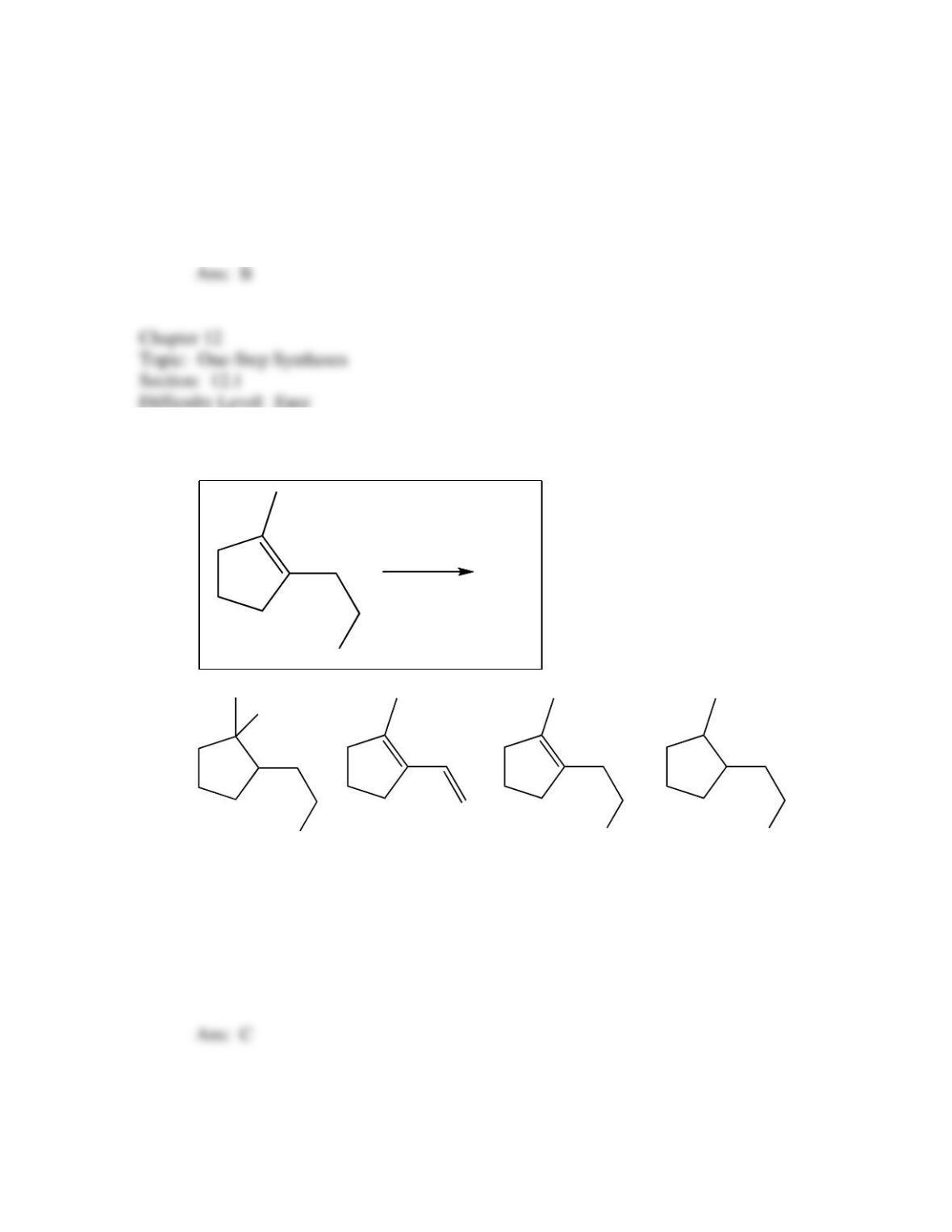
34. Predict the reagent(s) required to complete the following reaction: