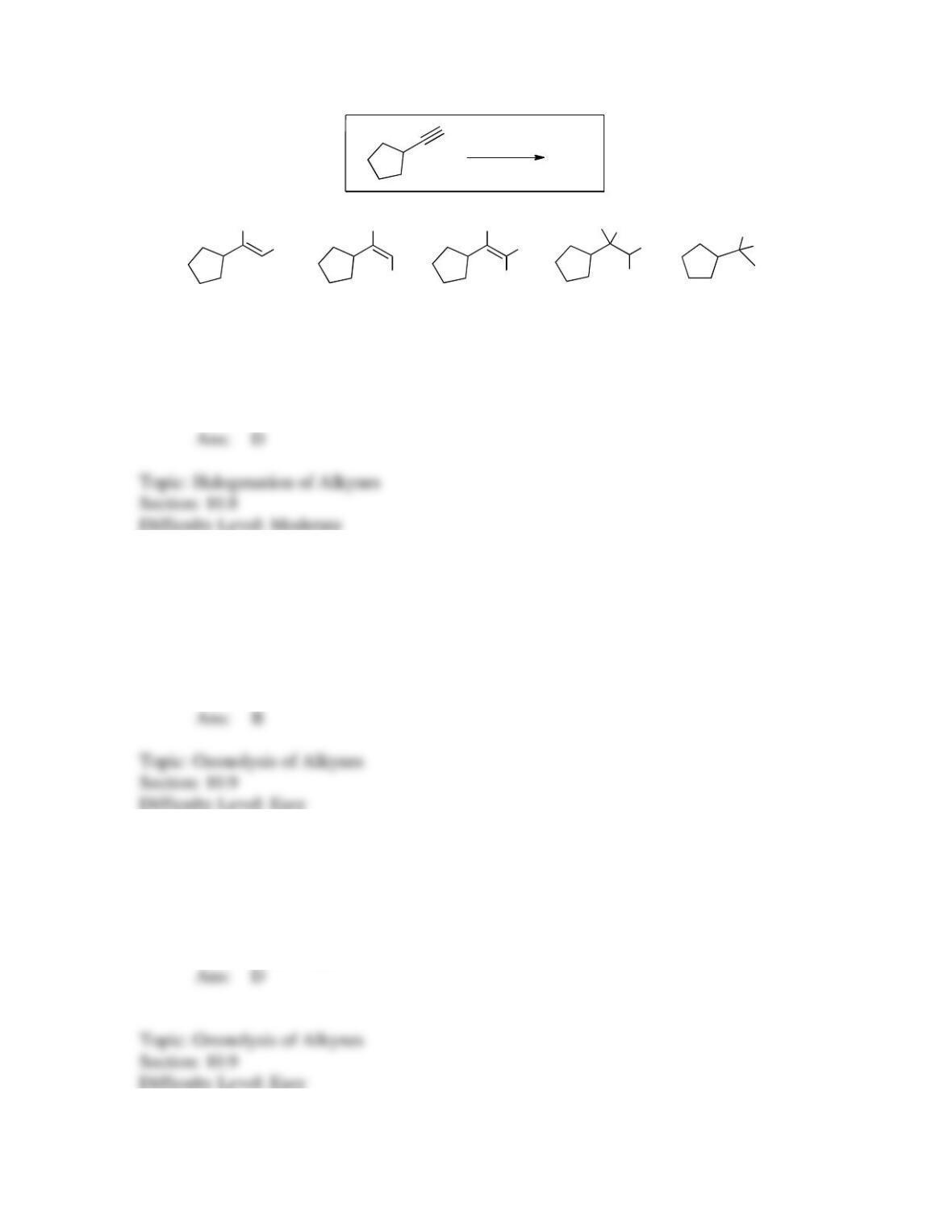
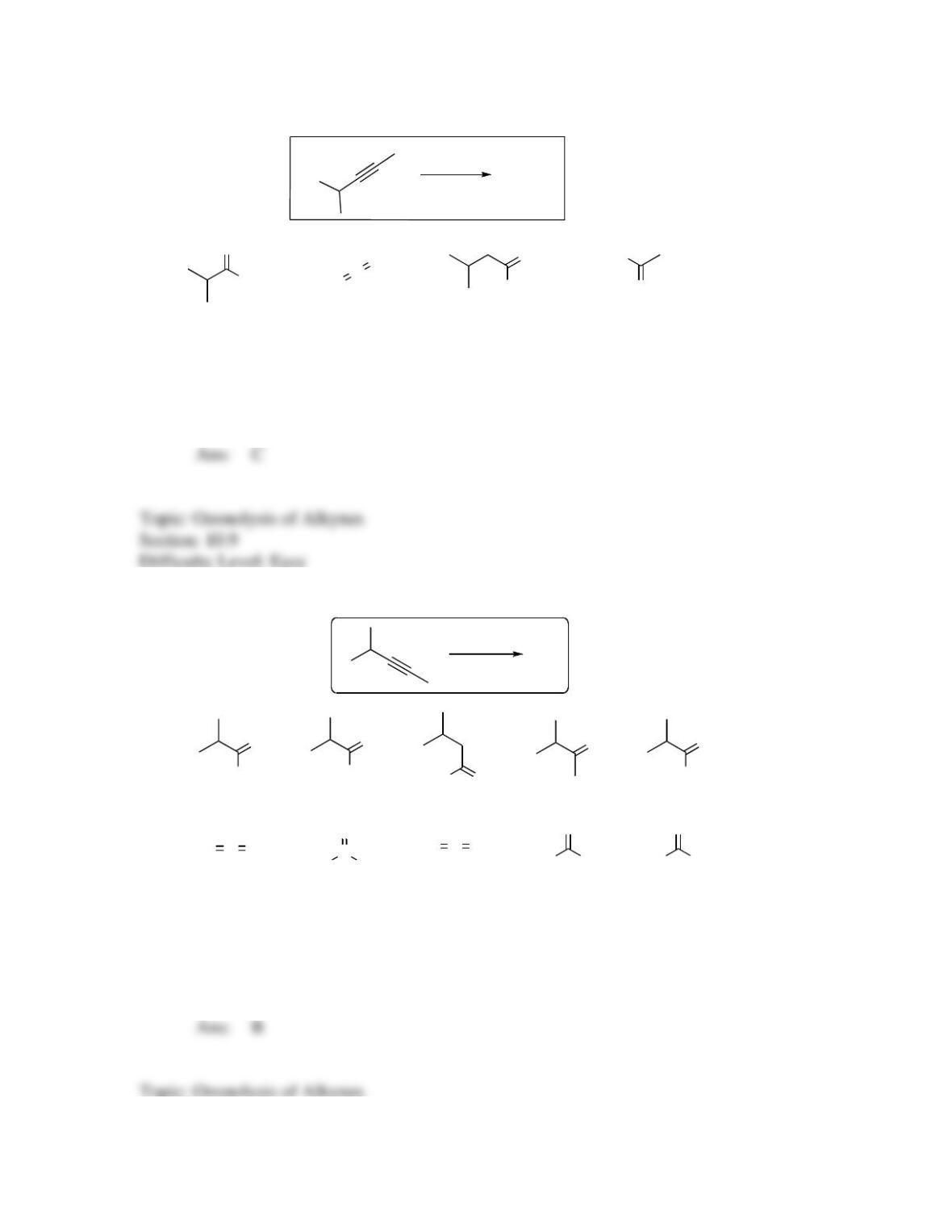
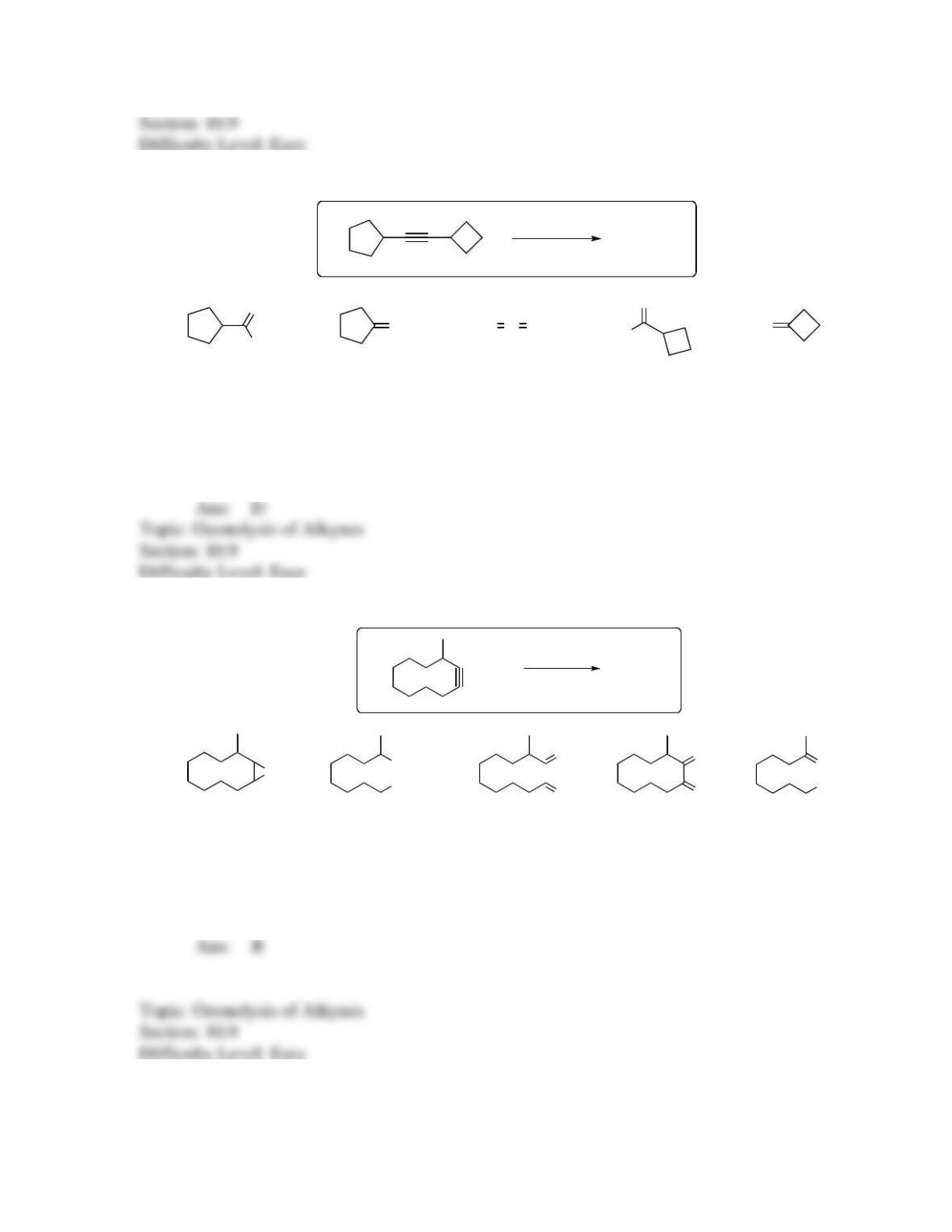
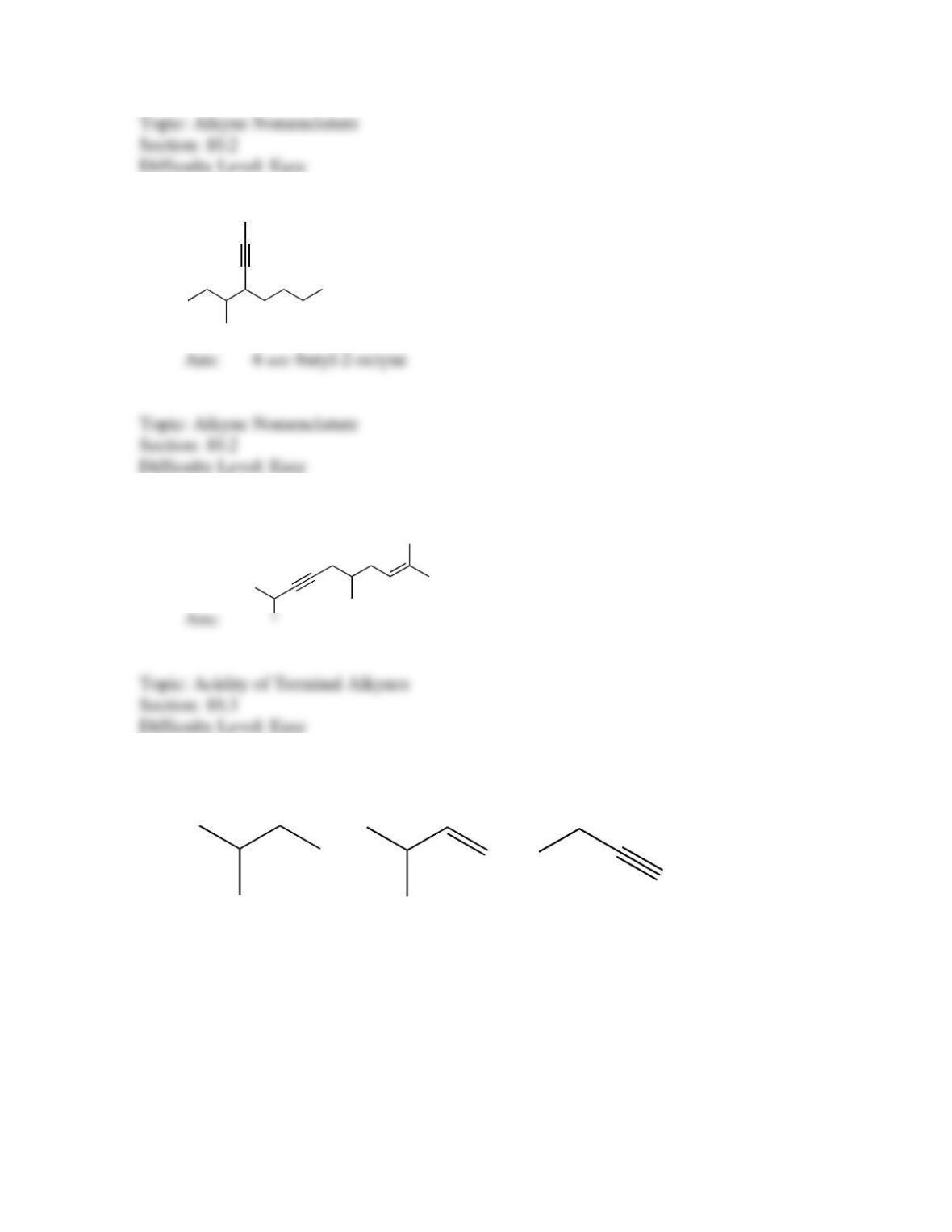
142. Which sequence of reactions is expected to produce the product below as the final,
and major, organic product?
CH3
OH
HO
H
H
CH3
?+ enantiomer
A) 1) Na, NH3(l); 2) OsO4; 3) NaHSO3/H2O
B) 1) H2, Lindlar’s cat.; 2) MCPBA; 3) H3O+
C) 1) H2, Ni2B (P-2); 2) KMnO4, NaOH (cold)
D) Both A and B
E) Both A and C
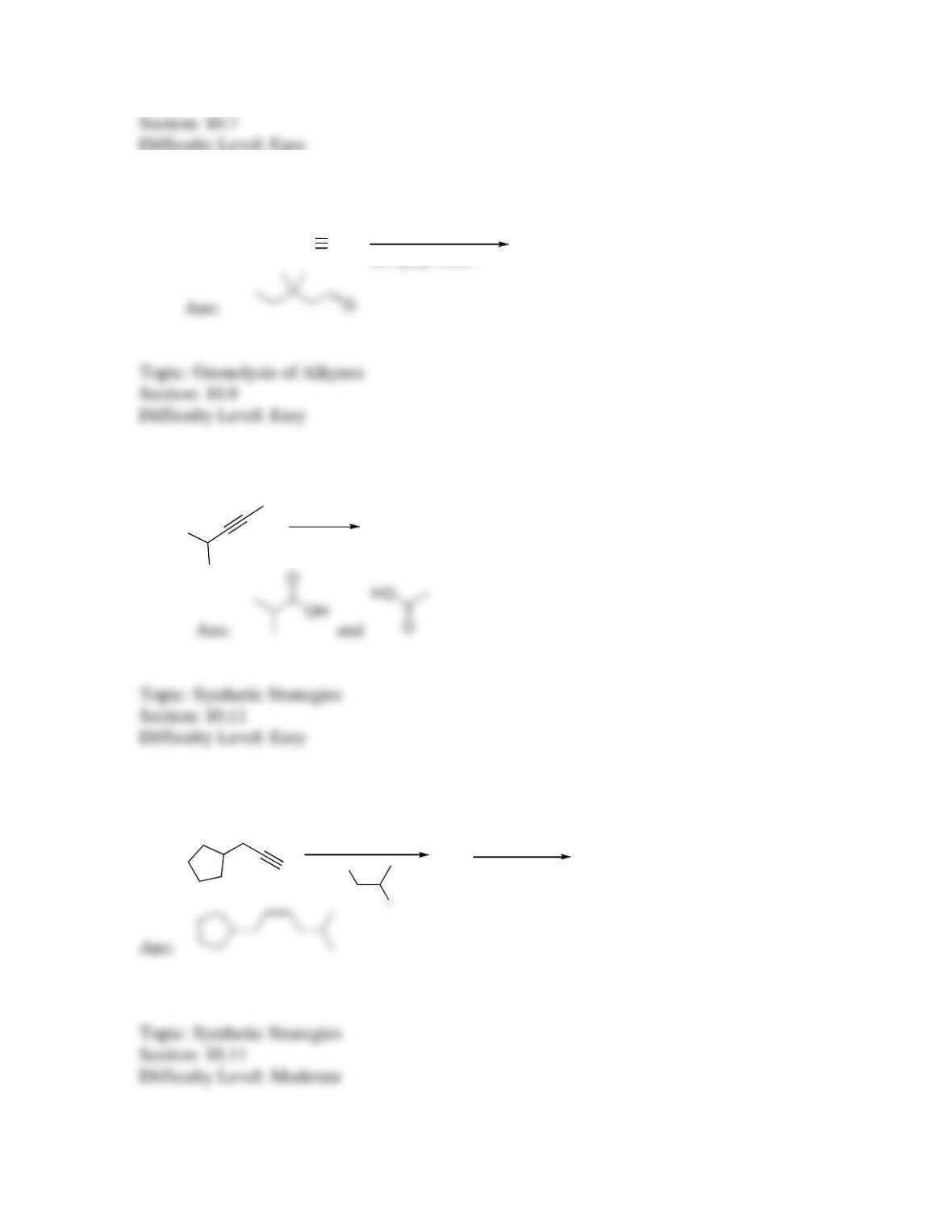
143. Which sequence of reactions is expected to produce the product below as the final,
and major, organic product?
CH2CH3
Br
Br
H
H
?+ enantiomer
A) 1) Br2; 2) H2, Lindlar’s cat.
B) 1) H2, Lindlar’s cat.; 2) Br2
C) 1) Br2; 2) Na, NH3(l)
D) 1) Na, NH3(l); 2) Br2
E) 1) Br2, 2) H2, Pt
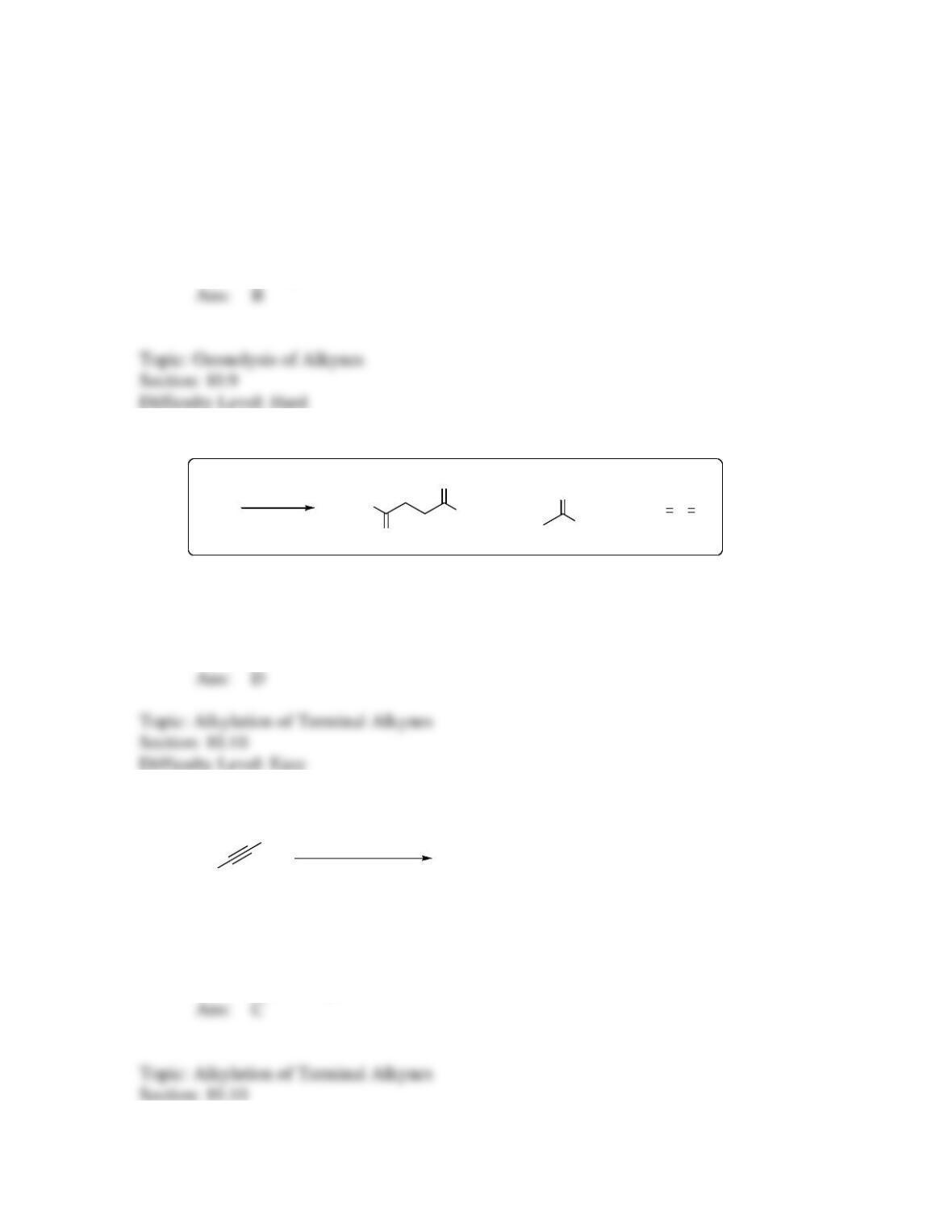
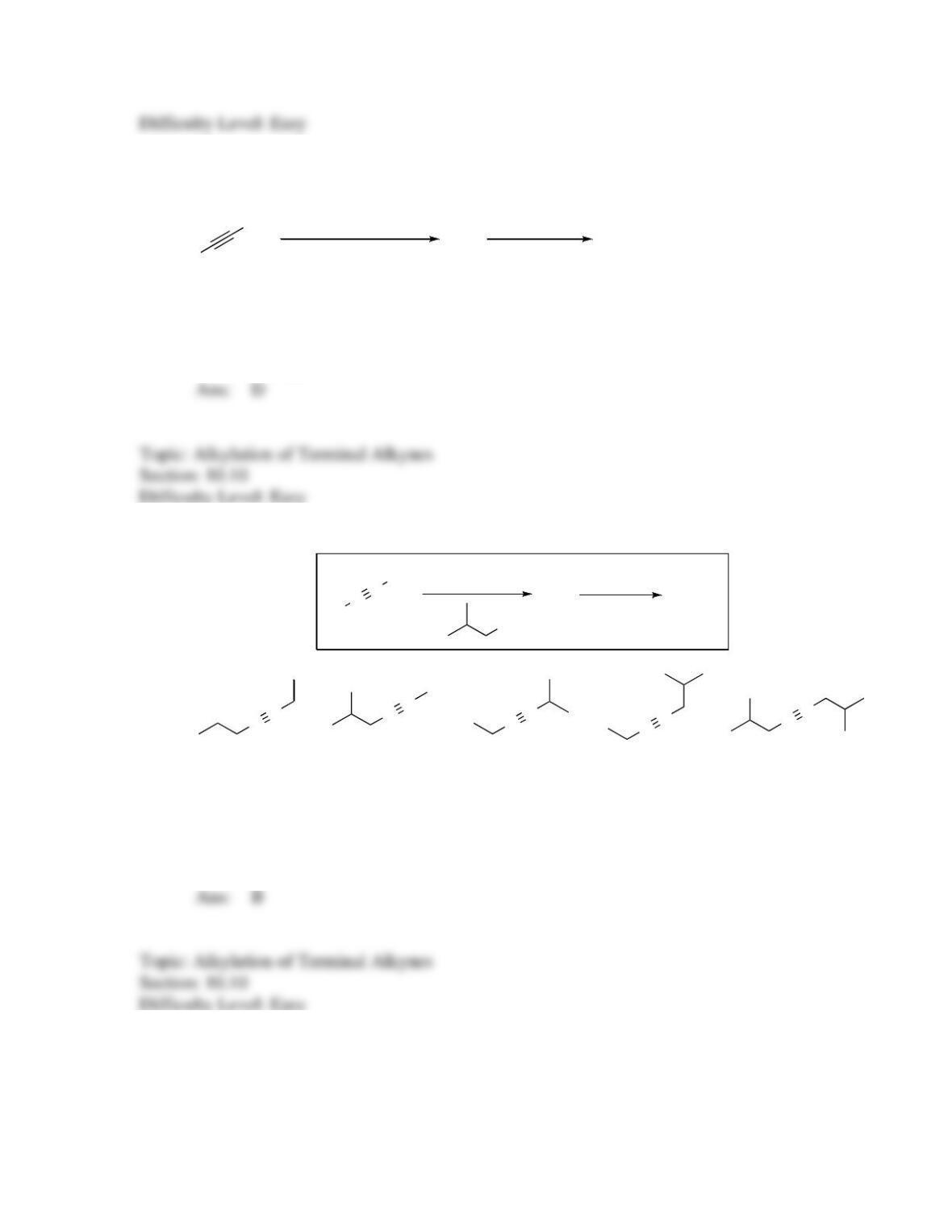
144. What is the final major product expected for the following reaction sequence?
A) (R)-3-Chlorohexane
B) (S)-3-Chlorohexane
C) meso-3,4-Dichlorohexane
D) Racemic (2R,3R) and (2S,3S)-3,4-dichlorohexane
E) 2,3-Dichloro-3-hexene