In reference to waves, frequency is the
A. number of waves passing a fixed point in one second.
B. height of the wave.
C. distance between successive peaks in a wave.
D. distance between a peak in a wave to the next trough.
The mass number of an isotope of an element is the
A. sum of the number of its protons and electrons.
B. number of its protons.
C. sum of the number of its protons and neutrons.
D. sum of the number of its protons, neutrons, and electrons.
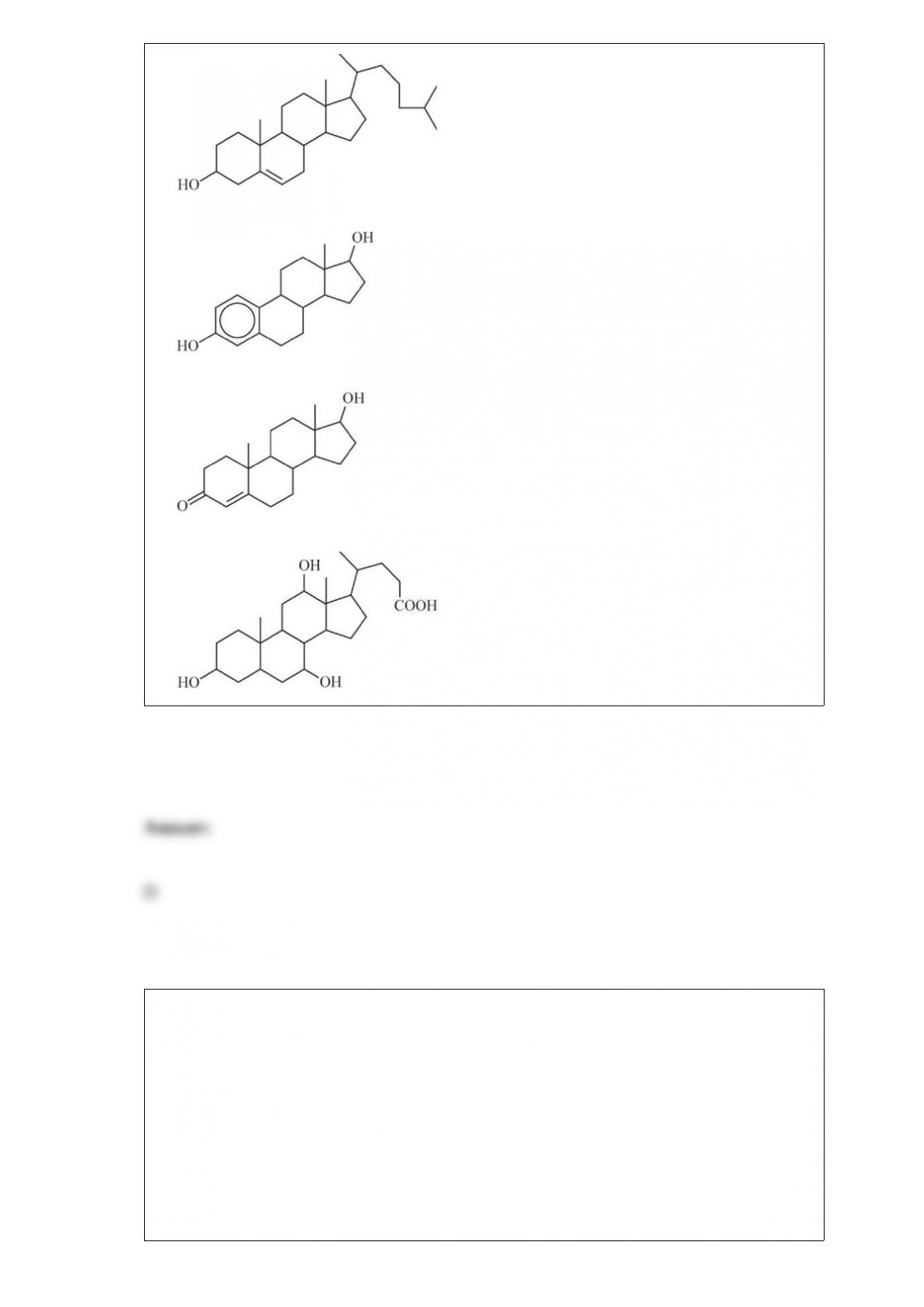
Some steroids are more soluble in water than others. Based on your understanding of
the solubility of molecular compounds in water, decide which compound is the most
soluble.