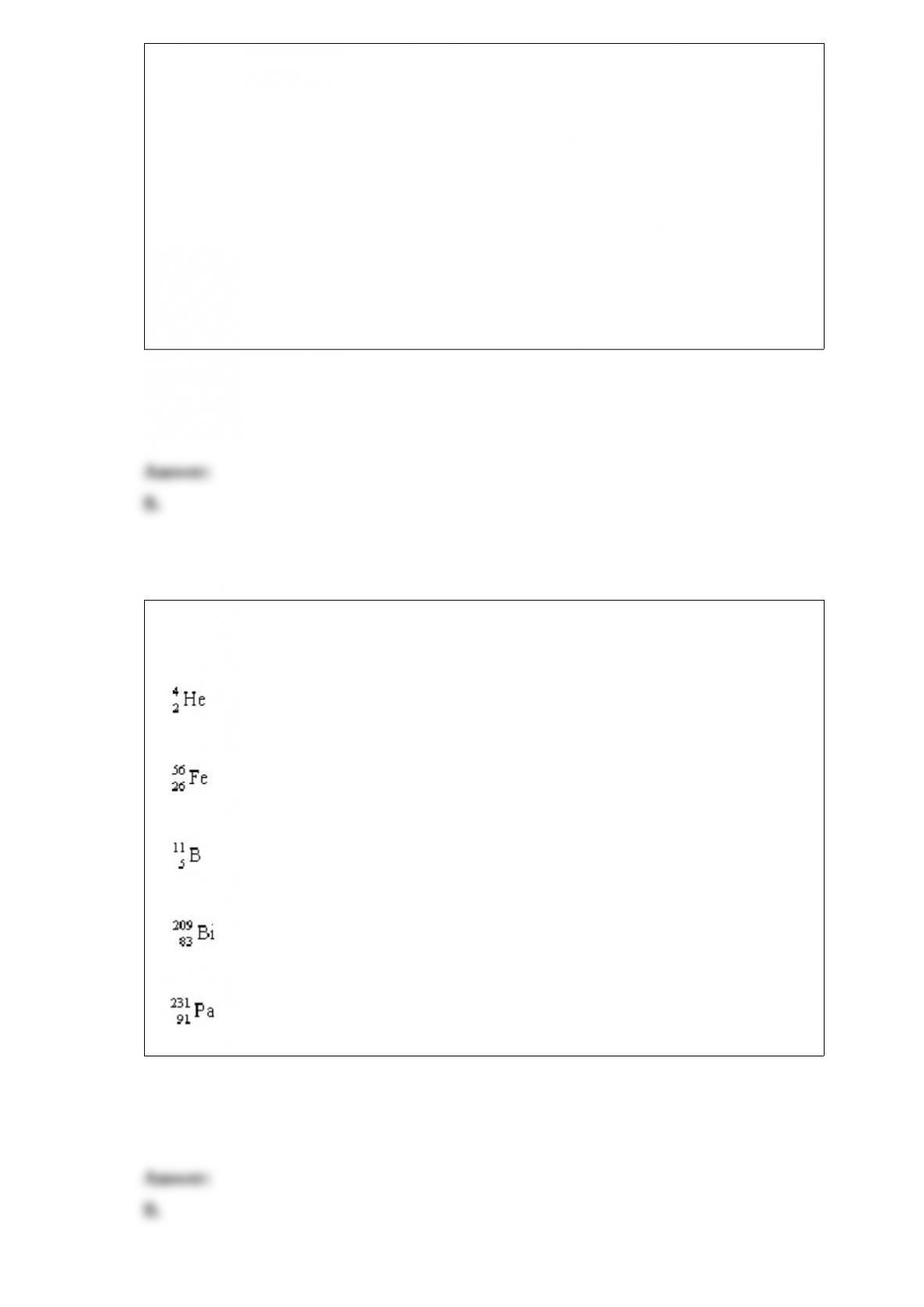
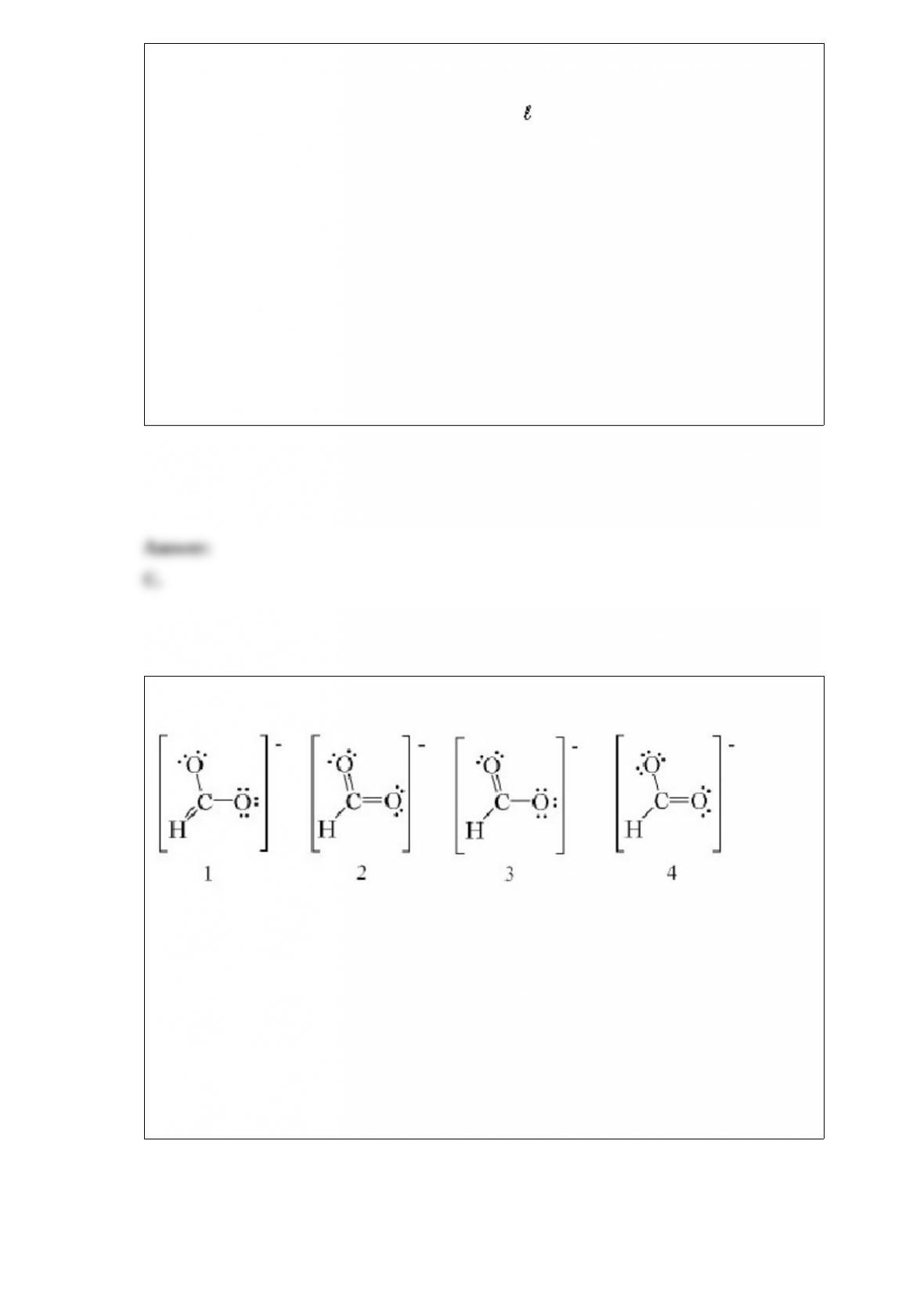
equations below.
Fe(s) + 3 H2O( ) Fe(OH)3(s) + 3/2 H2(g) DrH = +160.9 kJ/mol-rxn
H2(g) + 1/2 O2(g) H2O( ) DrH = "285.8 kJ/mol-rxn
Fe2O3(s) + 3 H2O( ) 2 Fe(OH)3(s) DrH = +288.6 kJ/mol-rxn
A."252.6 kJ/mol-rxn
B.+163.7 kJ/mol-rxn
C."824.2 kJ/mol-rxn
D.+33.2 kJ/mol-rxn
E.+ 890.6 kJ/mol-rxn
What is the mass percent of each element in sulfuric acid, H2SO4?
A.2.055% H, 32.69% S, 65.25% O
B.1.028% H, 32.69% S, 66.28% O
C.28.57% H, 14.29% S, 57.17% O
D.1.028% H, 33.72% S, 65.25% O
E.2.016% H, 32.07% S, 65.91% O