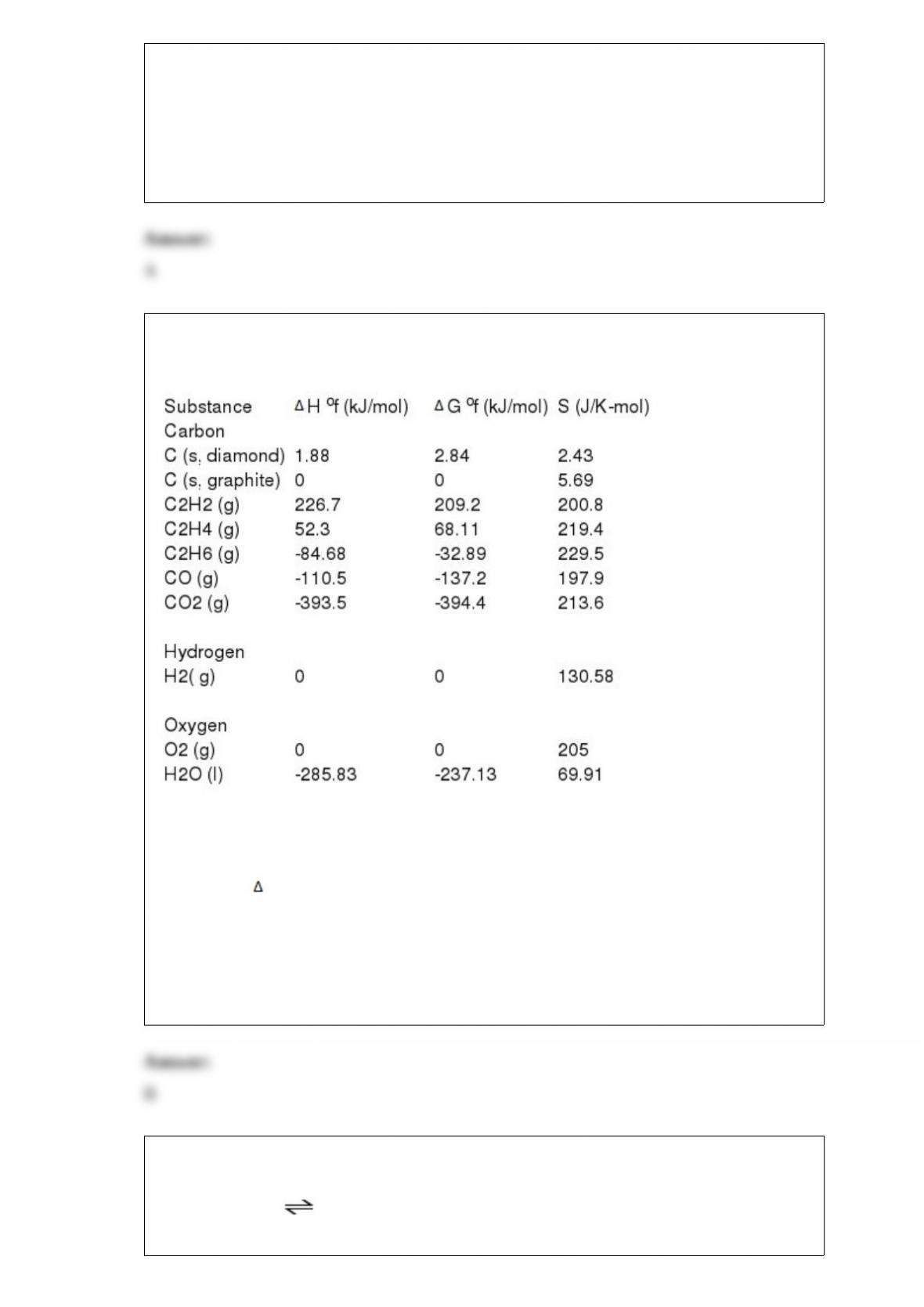
At equilibrium in a particular experiment, the concentrations of CO and H2 were 0.15
M and 0.36, M respectively. What is the equilibrium concentration of CH3OH? The
value of Keq for this reaction is 14.5 at the temperature of the experiment.
A) 14.5
B) 7.61 x 10-3
C) 2.82 x 10-1
D) 3.72 x 10-3
E) 1.34 x 10-3
15) Which equation correctly represents the reaction between silica and hydrofluoric
acid?
A) SiCl4 + 4HF --> SiF4 + 4HCl
B) SiO2 + 6HF --> H2SiF6 + 2H2O
C) SiCl2 + 2HF --> SiF2 + 2HCl
D) SiH4 + 4HF --> SiF4 + 4H2
E) none of the above
16) For a given arrangement of ions, the lattice energy increases as ionic radius
________ and as ionic charge ________.
A) decreases, increases
B) increases, decreases
C) increases, increases
D) decreases, decreases
E) This cannot be predicted.
17) The rate law of a reaction is rate = k[D][X]. The units of the rate constant are
________.
A) mol L-1s-1
B) L mol-1s-1
C) mol2 L-2s-1
D) mol L-1s-2
E) L2 mol-2s-1