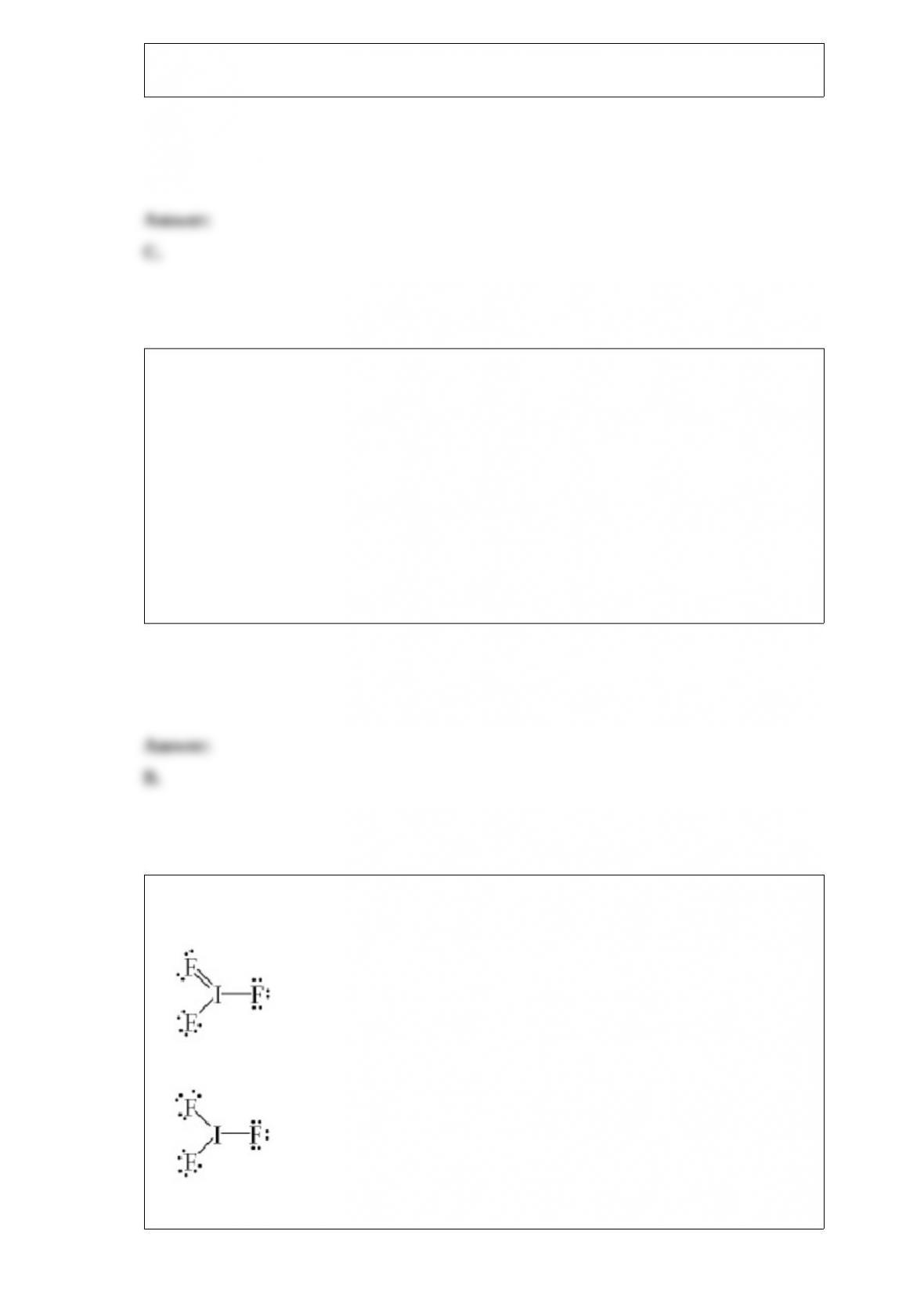
To form a molecule with an octahedral electron geometry, what set of pure atomic
orbitals must be mixed?
A.one s and three p
B.one s, three p, and one d
C.one s, three p, and two d
D.two s, six p, and two d
E.two s, six p, and four d
Which of the following statements concerning solubility is/are CORRECT?
1/ Ionic compounds composed of Group 1A metal ions and halide ions, such as NaCl,
are insoluble in nonpolar solvents.
2/ The solubility of the halogens (Cl2, Br2, and I2) in polar solvents is greater than their
solubility in nonpolar solvents.
3/ The solubility of polar molecules, such as sugar, in polar solvents is generally greater
than their solubility in nonpolar solvents.
A.1 only
B.2 only
C.3 only
D.1 and 3
E.1, 2, and 3