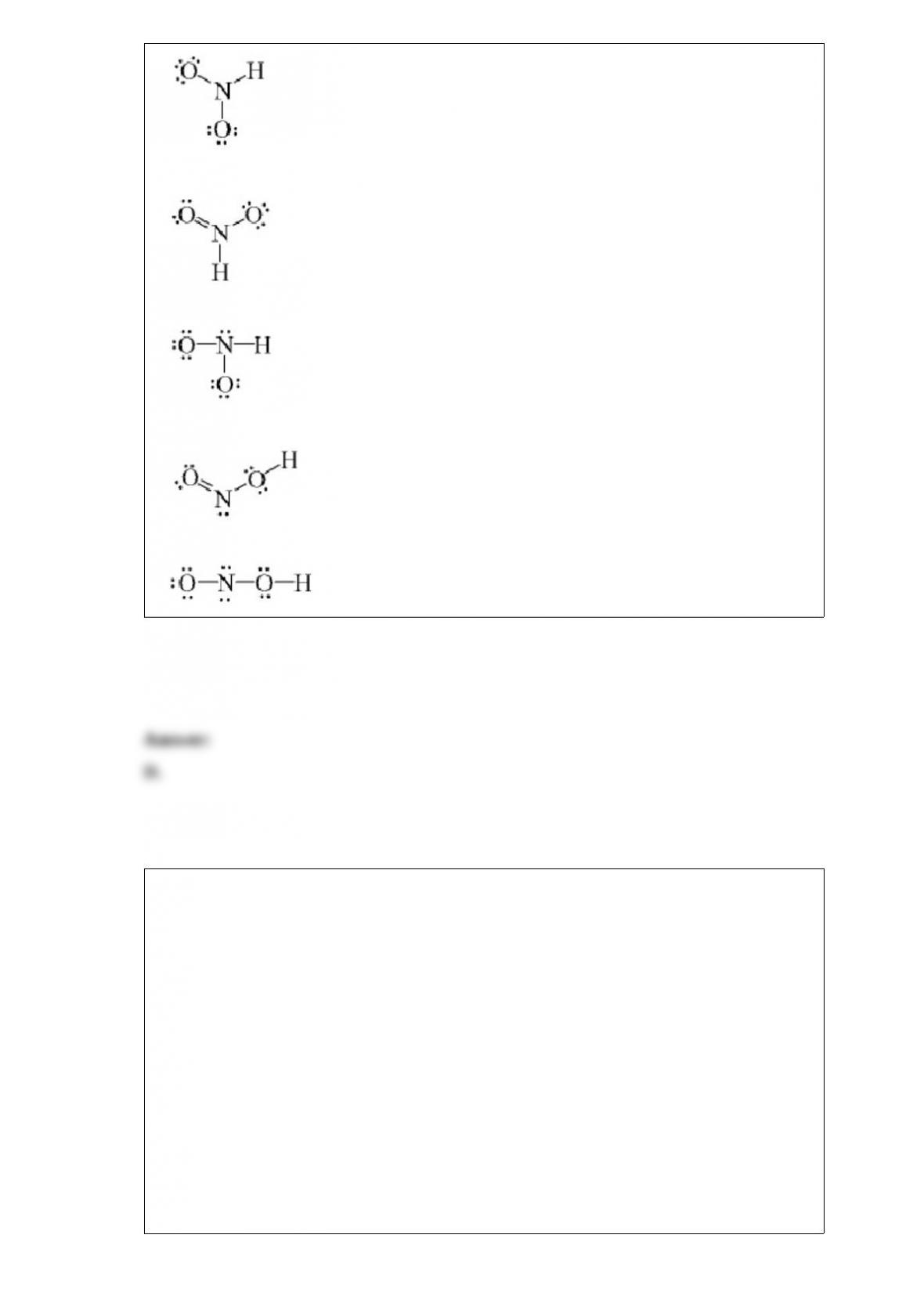
B.DrH > 0, DrS > 0, DrG > 0
C.DrH > 0, DrS > 0, DrG < 0
D.DrH < 0, DrS < 0, DrG < 0
E.DrH < 0, DrS > 0, DrG > 0
Which of the following statements is/are CORRECT?
1/ FM radio waves have longer wavelengths than visible radiation and AM radio waves
have shorter wavelengths than visible radiation.
2/ Magnetic resonance imaging (MRI) uses a mixture of x-rays and gamma rays.
3/ Exposure to ultraviolet (UV) radiation can lead to sunburns.
A.1 only
B.2 only
C.3 only
D.1 and 2
E.1, 2, and 3
Molecules must overcome a barrier called the activation energy if they are to react. The
highest energy point reached during the progress of a reaction is called the ____.