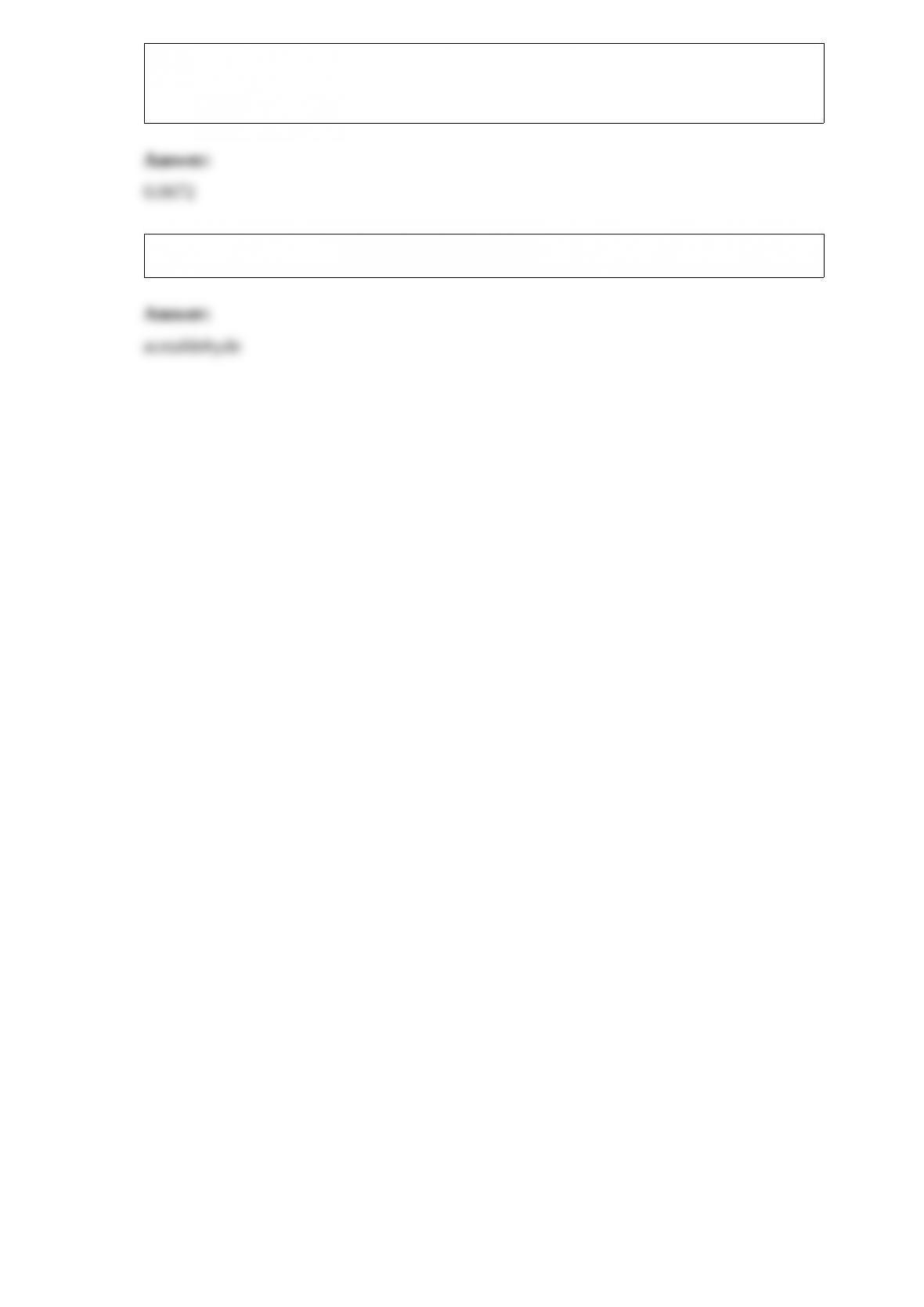
13) The output of a plant is 4335 pounds of ball bearings per week (five days). If each
ball bearing weighs 0.0113 g, how many ball bearings does the plant make in a single
day? (Indicate the number in proper scientific notation with the appropriate number of
significant figures.)
A) 3.84 X 105
B) 7.67 X 104
C) 867
D) 3.48 X 107
E) 2.91 X 106
14) A solution contains 30 ppm of benzene. The density of the solution is 1.00 g/mL.
This means that ________.
A) there are 30 mg of benzene in 1.0 L of this solution
B) 100 g of the solution contains 30 g of benzene
C) 100 g of the solution contains 30 mg of benzene
D) the solution is 30% by mass of benzene
E) the molarity of the solution is 30 M
15) What is the frequency (s-1) of a photon that has an energy of 4.38 X 10-18 J?
A) 436 s-1
B) 6.61 X 1015 s-1
C) 1.45 X 10-16 s-1
D) 2.30 X107 s-1
E) 1.31 X 10-9 s-1
16) A molecular formula always indicates ________.
A) how many of each atom are in a molecule
B) the simplest whole-number ratio of different atoms in a compound
C) which atoms are attached to which in a molecule
D) the isotope of each element in a compound
E) the geometry of a molecule