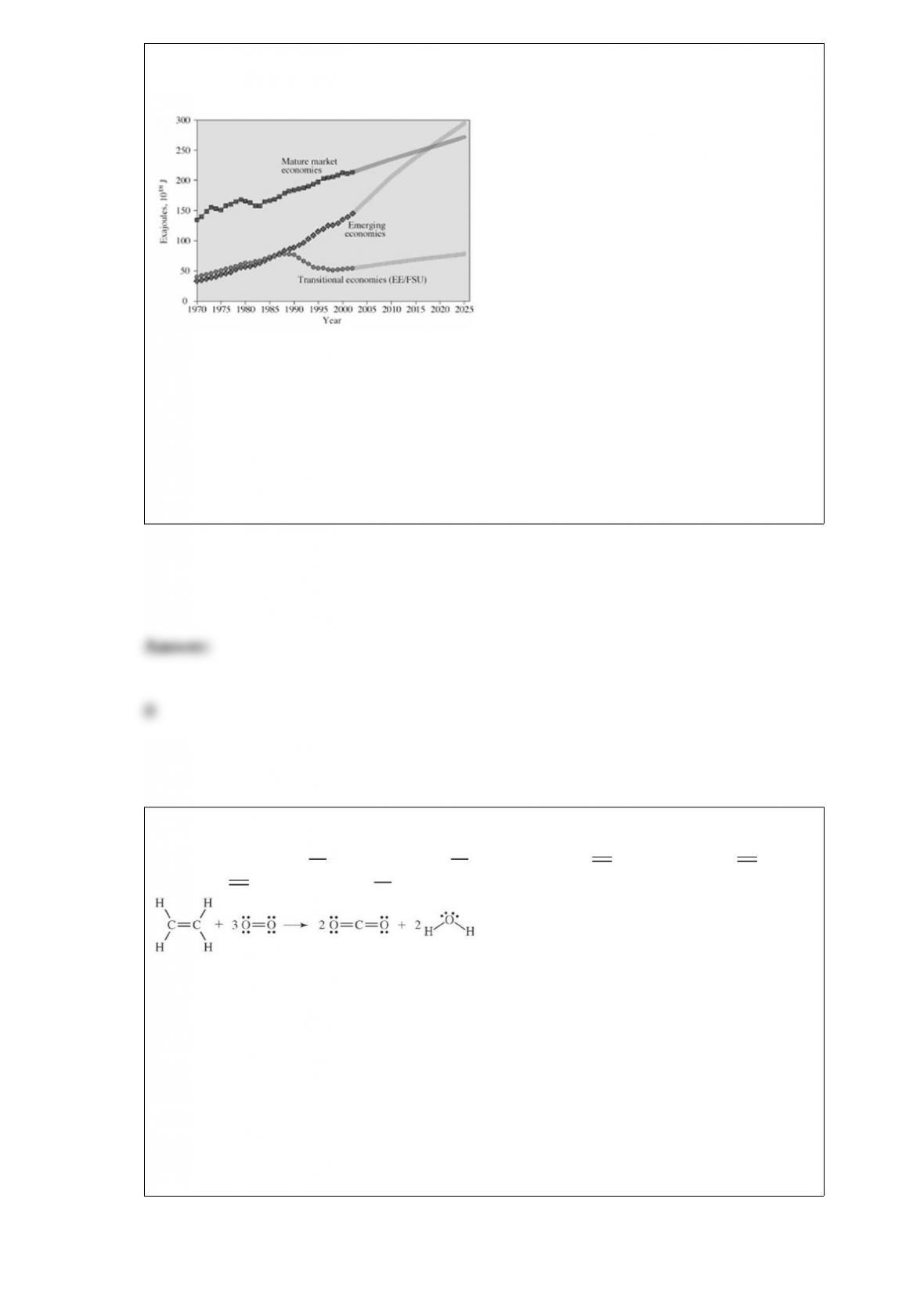
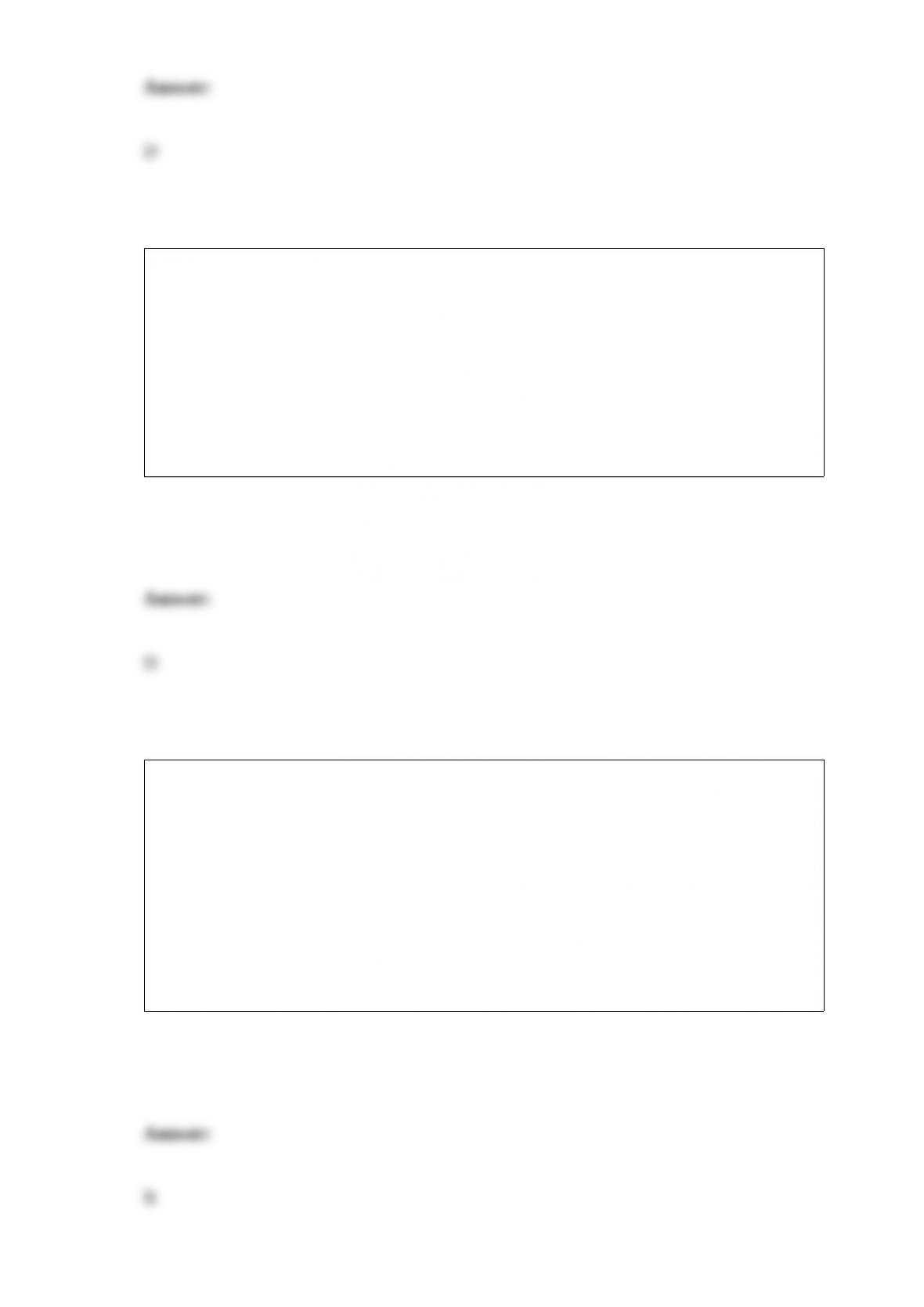
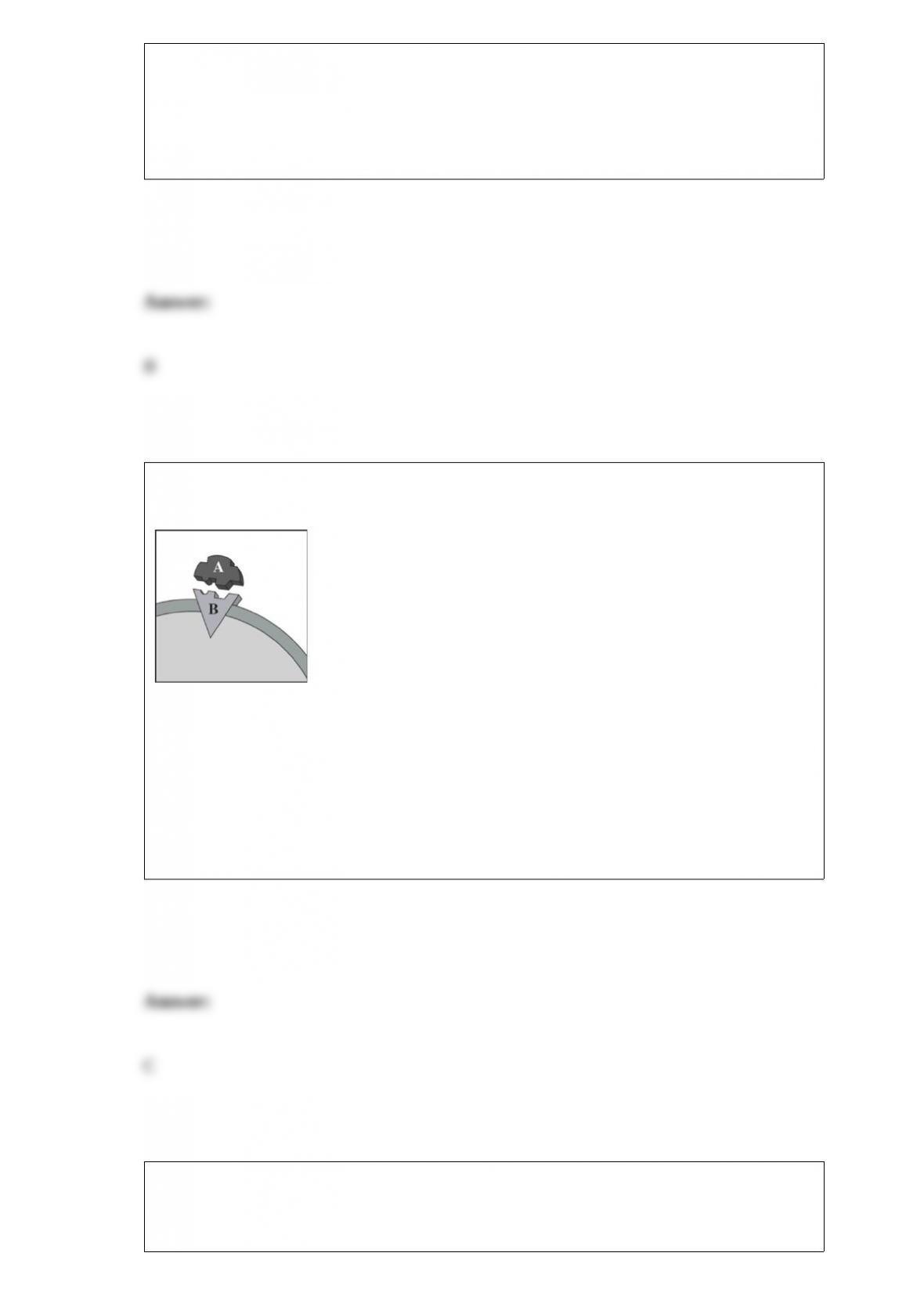
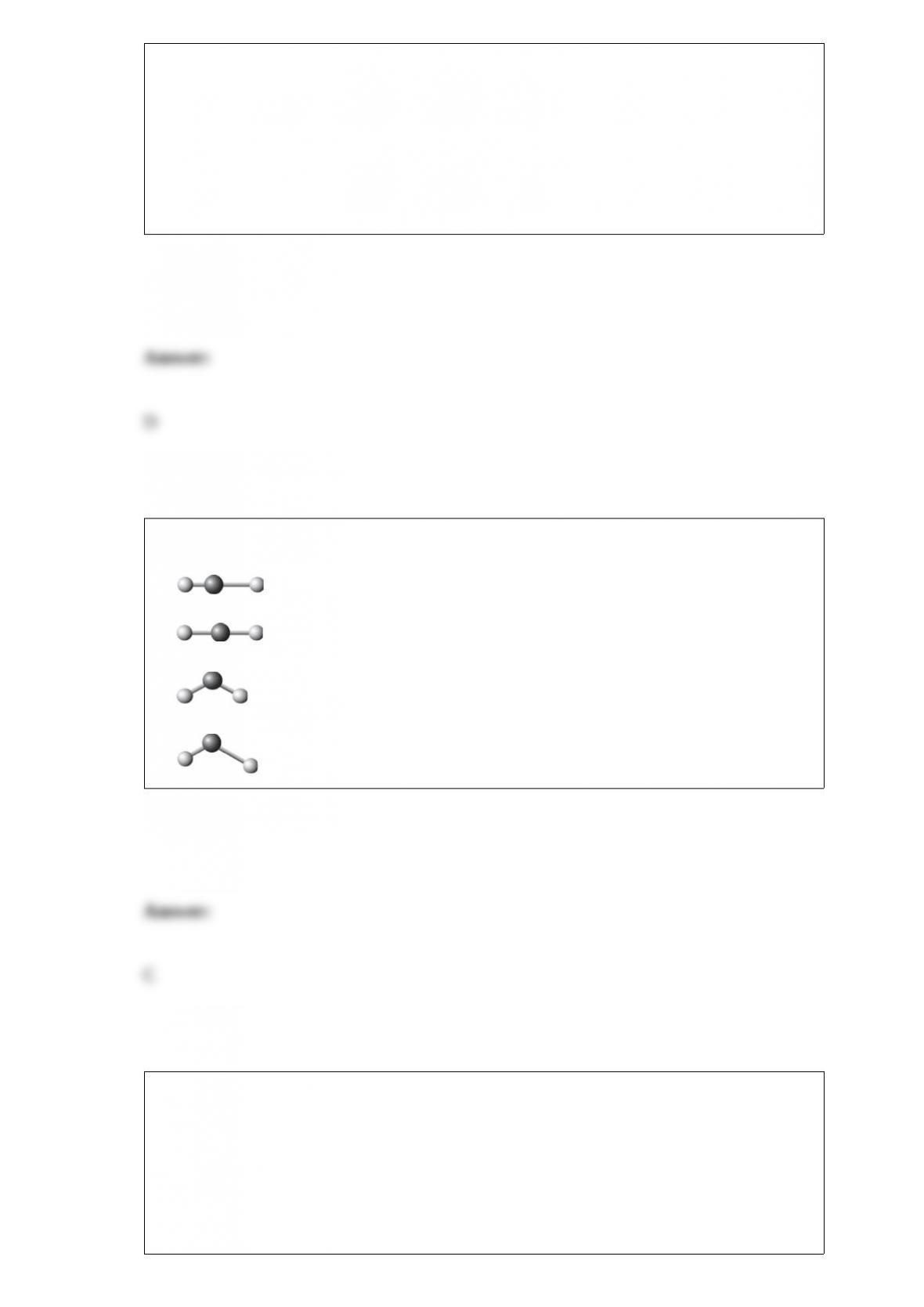
D. All of these choices are correct.
Which is not true of radioactive half-life? Radioactive half-life is
A. the time required for the level of radioactivity in a sample to be cut in half.
B. independent of the amount of radioactive material present.
C. increased by heating the isotope.
D. independent of the physical or chemical form of the isotope.
Although all aspirin tablets contain the same active ingredient, acetylsalicylic acid, not
all aspirin tablets are the same. Which is not a possible reason for this difference?
A. They contain fillers and bonding agents that may vary, depending on the
manufacturer.
B. Some may contain weak bases that act as buffers to reduce the natural acidity of the
aspirin.
C. Some may be coated to keep them intact until they reach the intestine.
D. Some may contain the inactive optical isomer in the preparation.