B) Fluoride stimulates production of tooth enamel to replace that lost to decay.
C) Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D) Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E) Fluoride dissolves plaque, reducing its decaying contact with teeth.
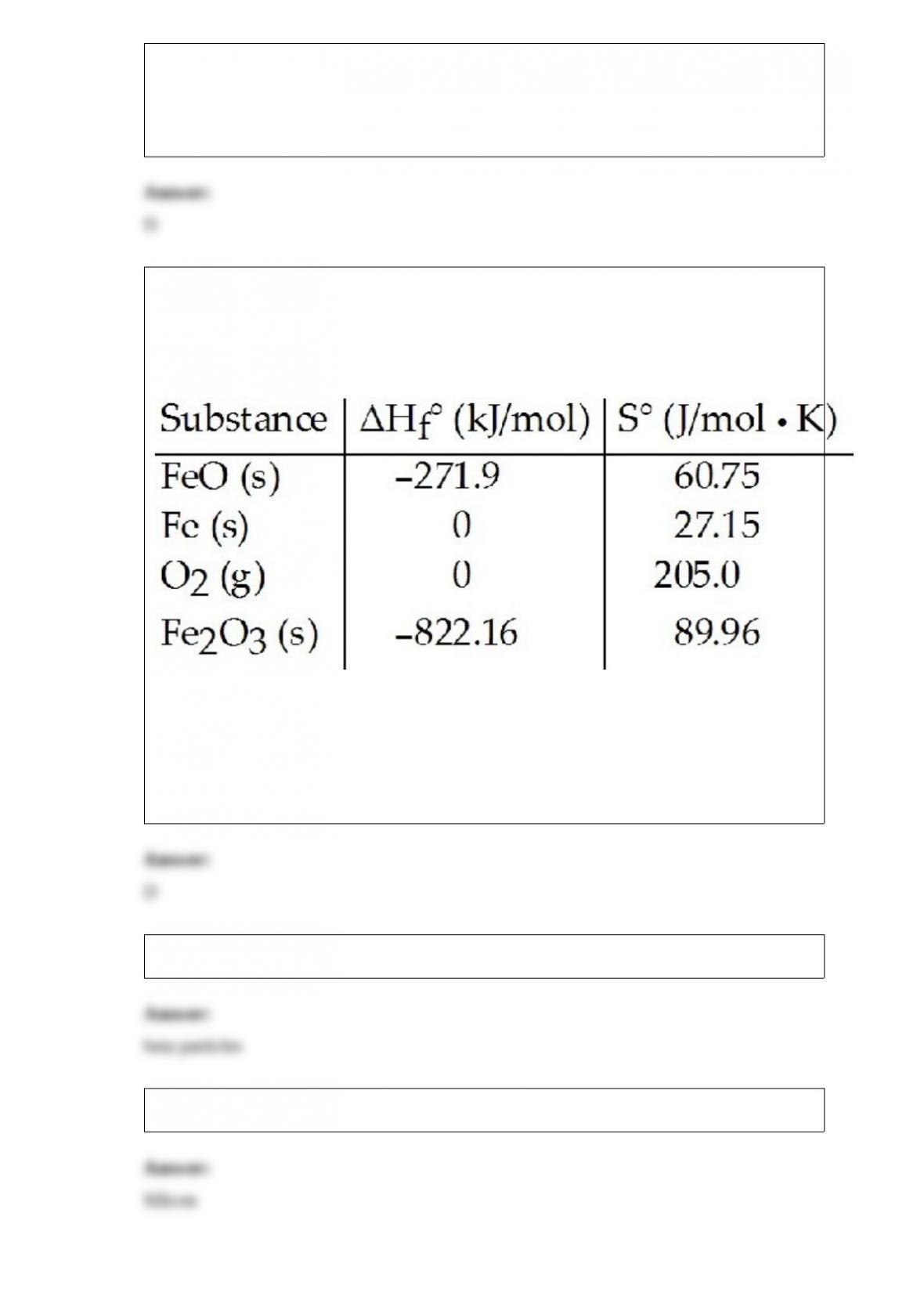
17) Consider the reaction:
FeO (s) + Fe (s) + O2 (g) --> Fe2O3 (s)
Given the following table of thermodynamic data,
determine the temperature (in oC) above which the reaction is nonspontaneous.
A) This reaction is spontaneous at all temperatures.
B) 618.1
C) 756.3
D) 2438
E) 1235
18) High speed electrons emitted by an unstable nucleus are called ________.
19) Si is the symbol for the element ________.