Which common process on earth is endothermic?
A. the production of oxygen by photosynthesis
B. ozone decomposing into O2 in the upper atmosphere
C. water freezing to form ice at 0C
D. the reaction of wood with oxygen to form carbon dioxide, water, and ash
Single bonds, double bonds, and triple bonds
A. have 1, 2, and 3 shared electrons, respectively.
B. have 2, 4, and 6 shared electrons, respectively.
C. have 3, 6, and 9 shared electrons, respectively.
D. are only possible between carbon atoms.
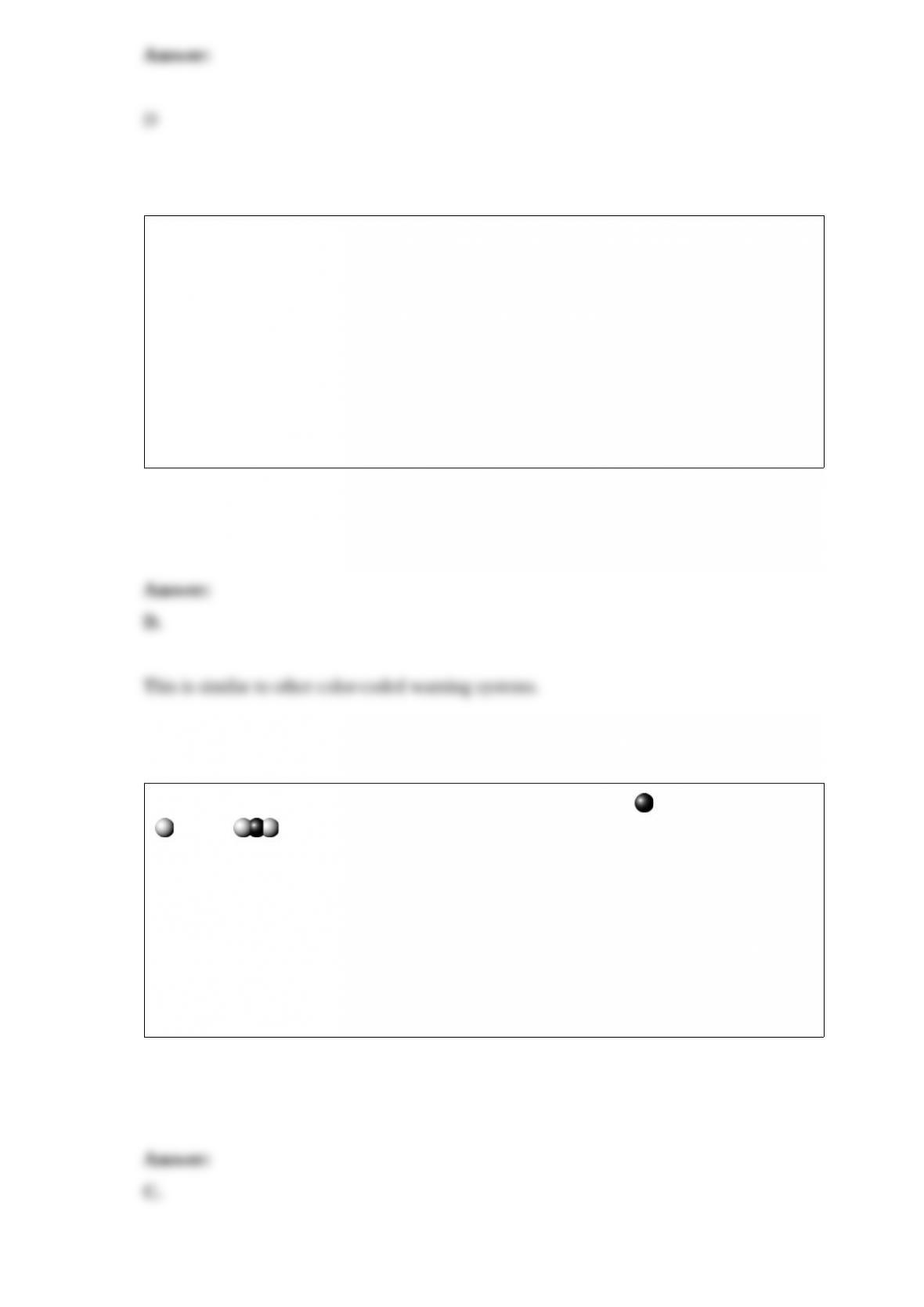
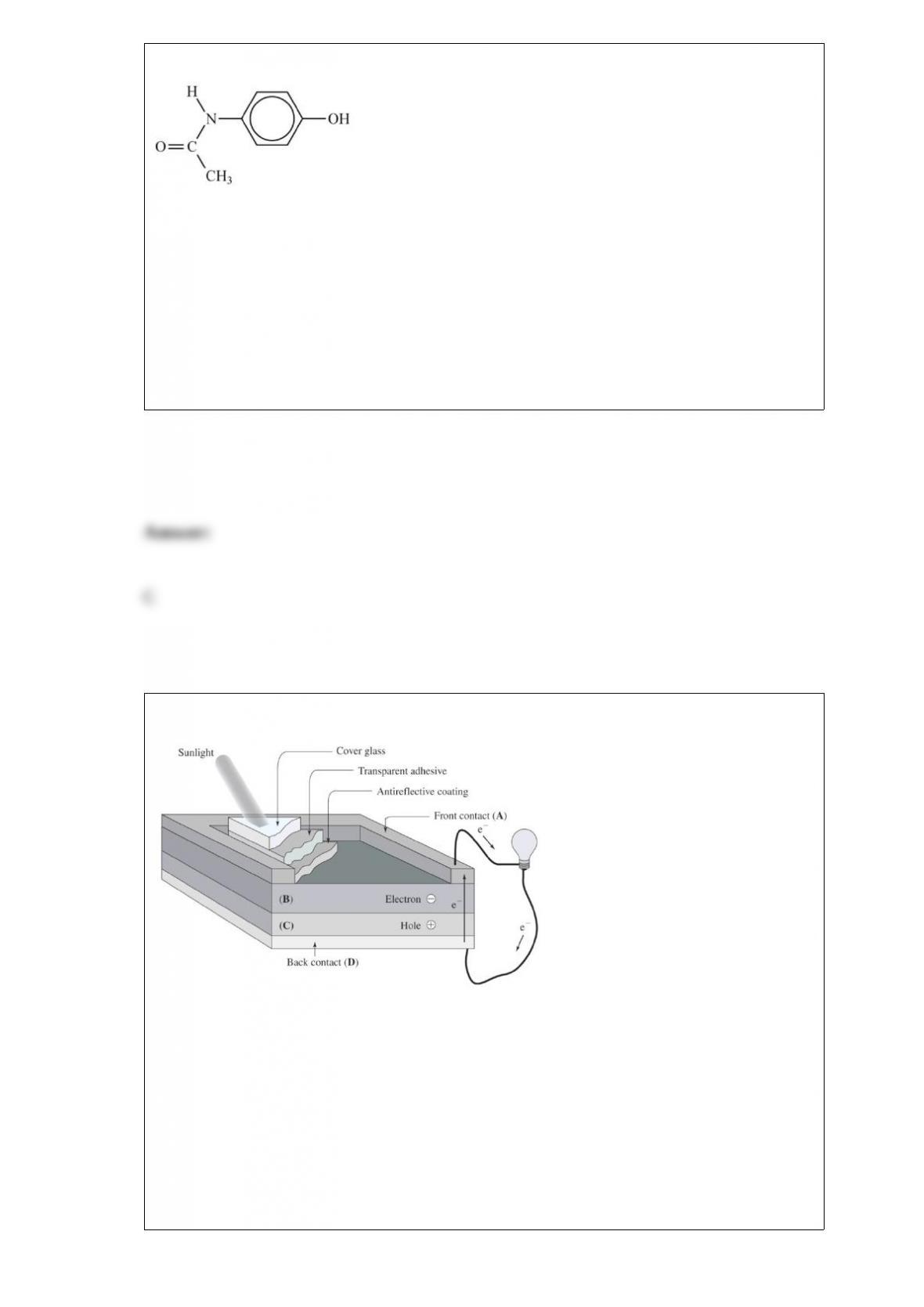
This is the condensed structural formula for acetaminophen, the active ingredient in the