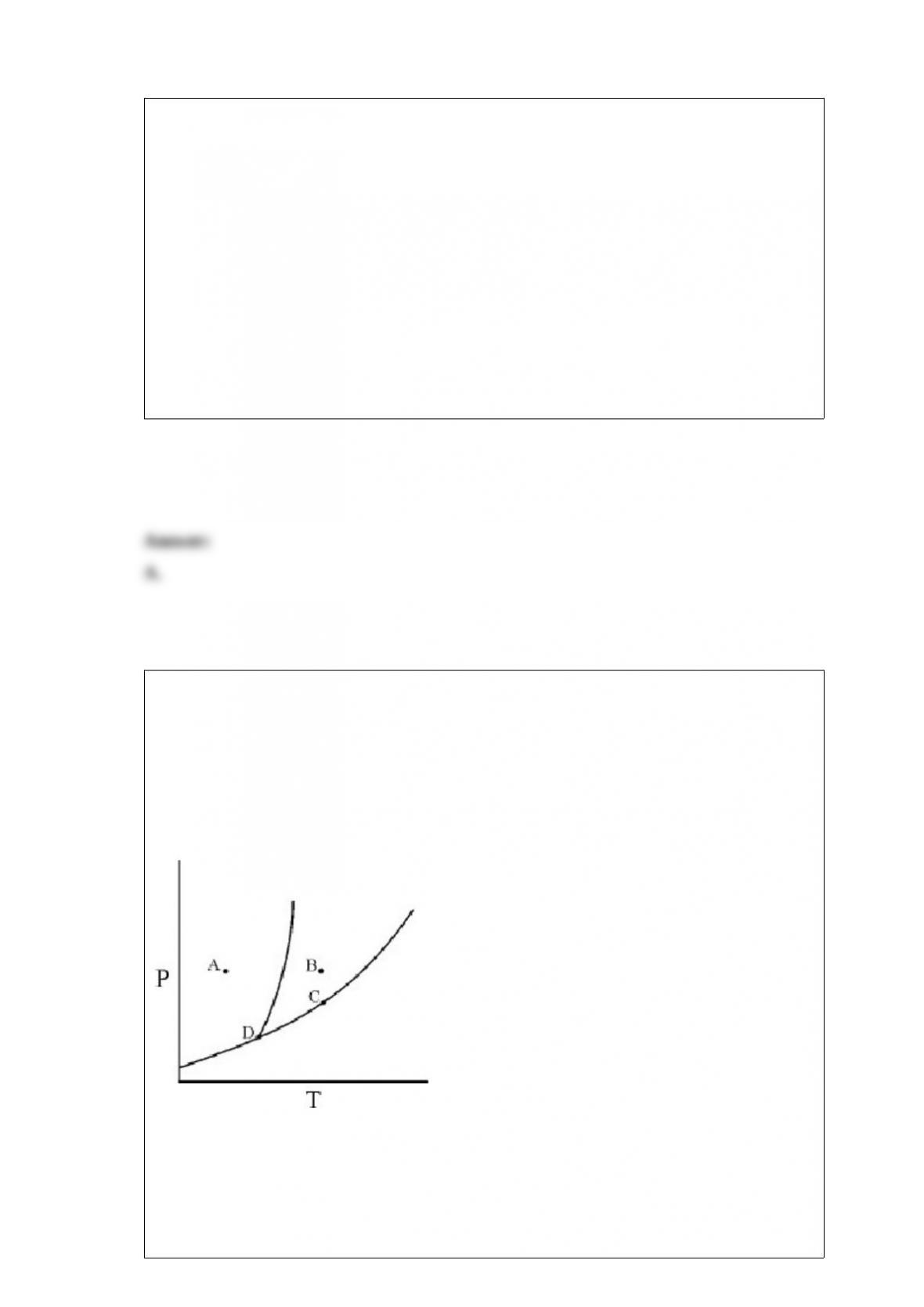
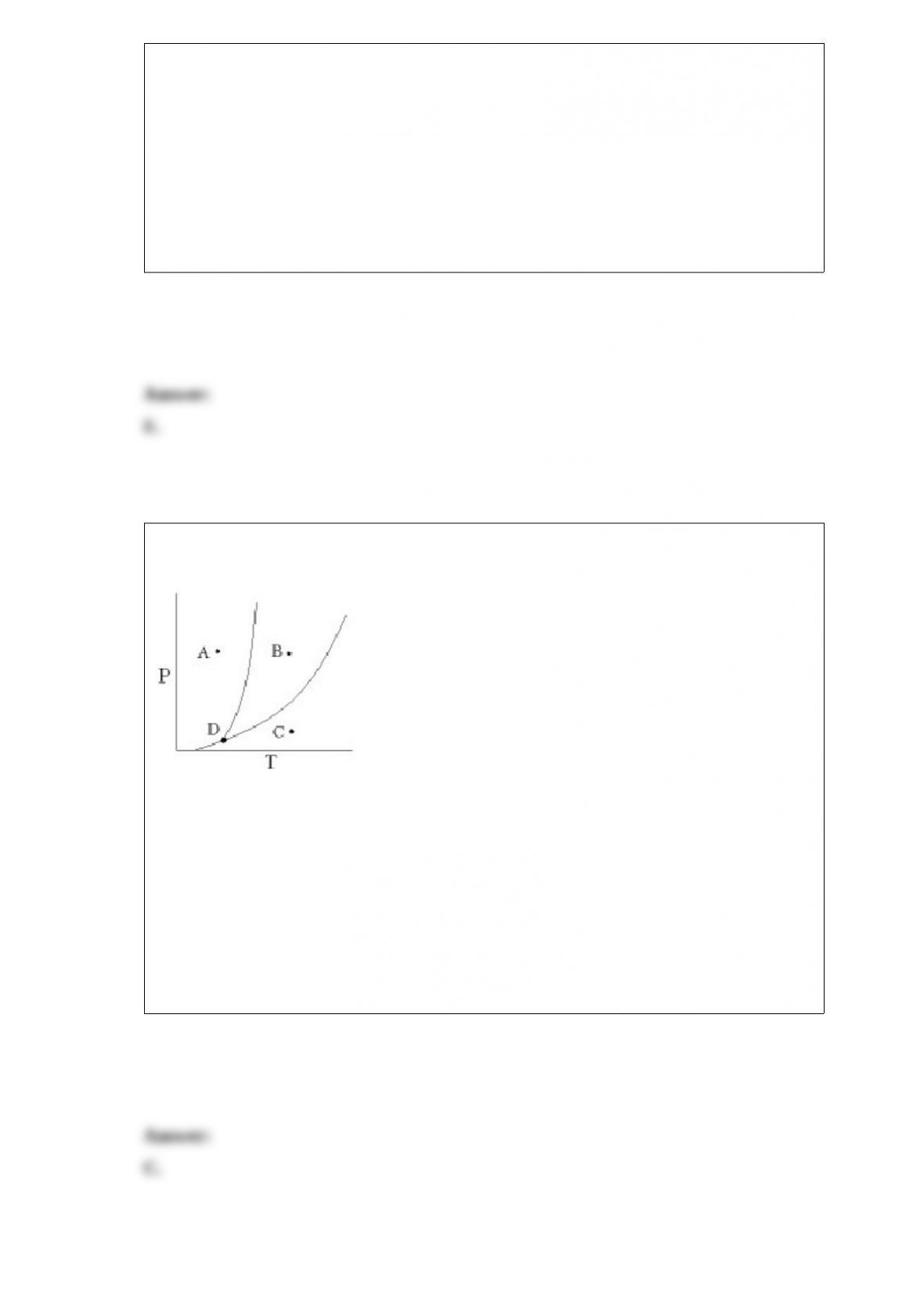
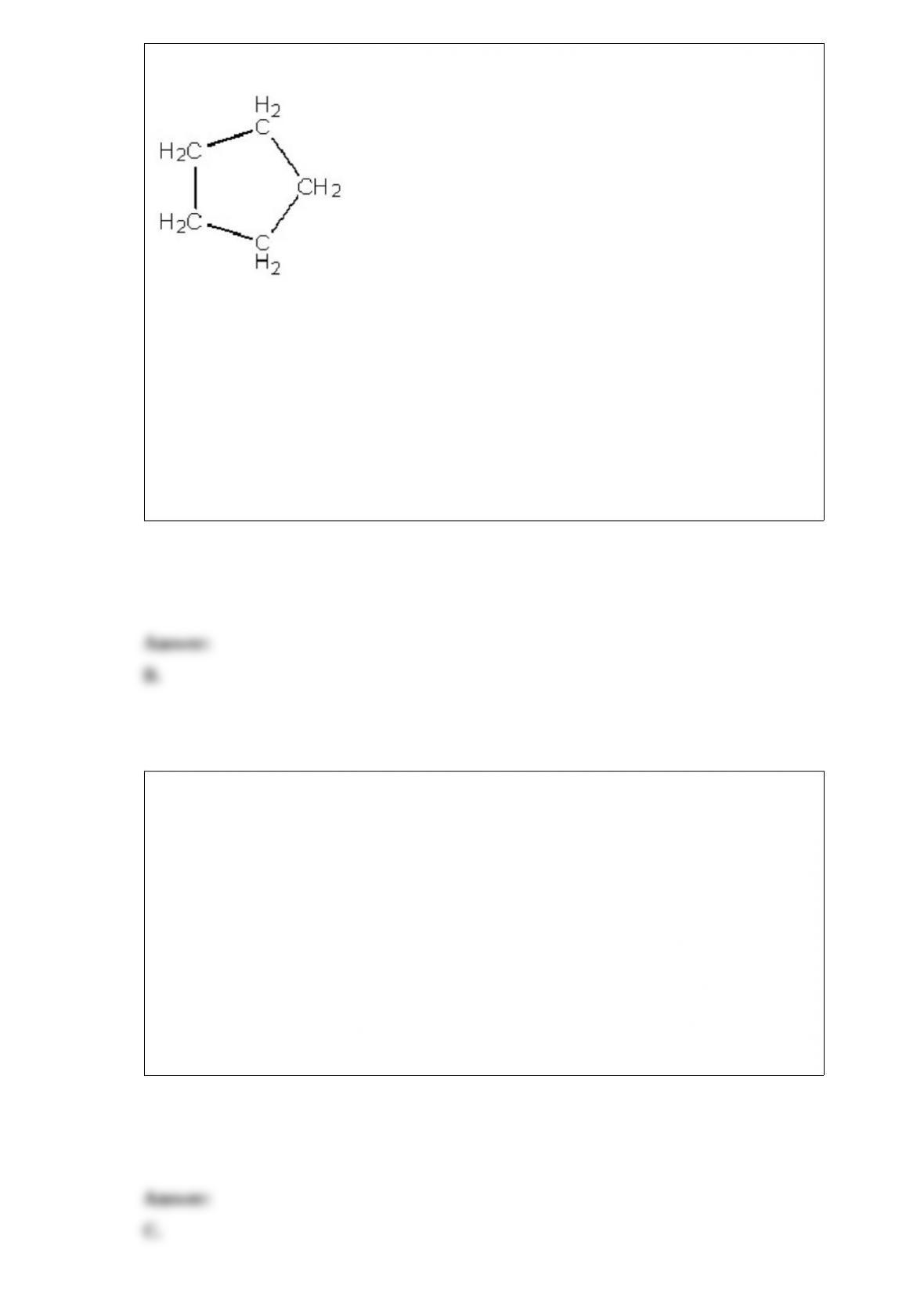
A.H3PO4 and H2PO4
", Ka1 = 7.5 10"3
B.HNO2 and NO2
", Ka = 4.5 10"4
C.CH3CO2H and CH3COO", Ka = 1.8 10"5
D.H2PO4
" and HPO4
2", Ka2 = 6.2 10"8
E.NH4
+ and NH3, Ka = 5.7 10"10
A 2.5 L flask is filled with 0.25 mol SO3, 0.20 mol SO2, and 0.40 mol O2, and allowed
to reach equilibrium. Assume the temperature of the mixture is chosen so that Kc =
0.12. Predict the effect on the concentration of SO3 as equilibrium is achieved by using
Q, the reaction quotient.
2 SO3(g) 2 SO2(g) + O2(g)
A.[SO3] will decrease because Q > K.
B.[SO3] will decrease because Q < K.
C.[SO3] will increase because Q < K.
D.[SO3] will increase because Q > K.
E.[SO3] will remain the same because Q = K.