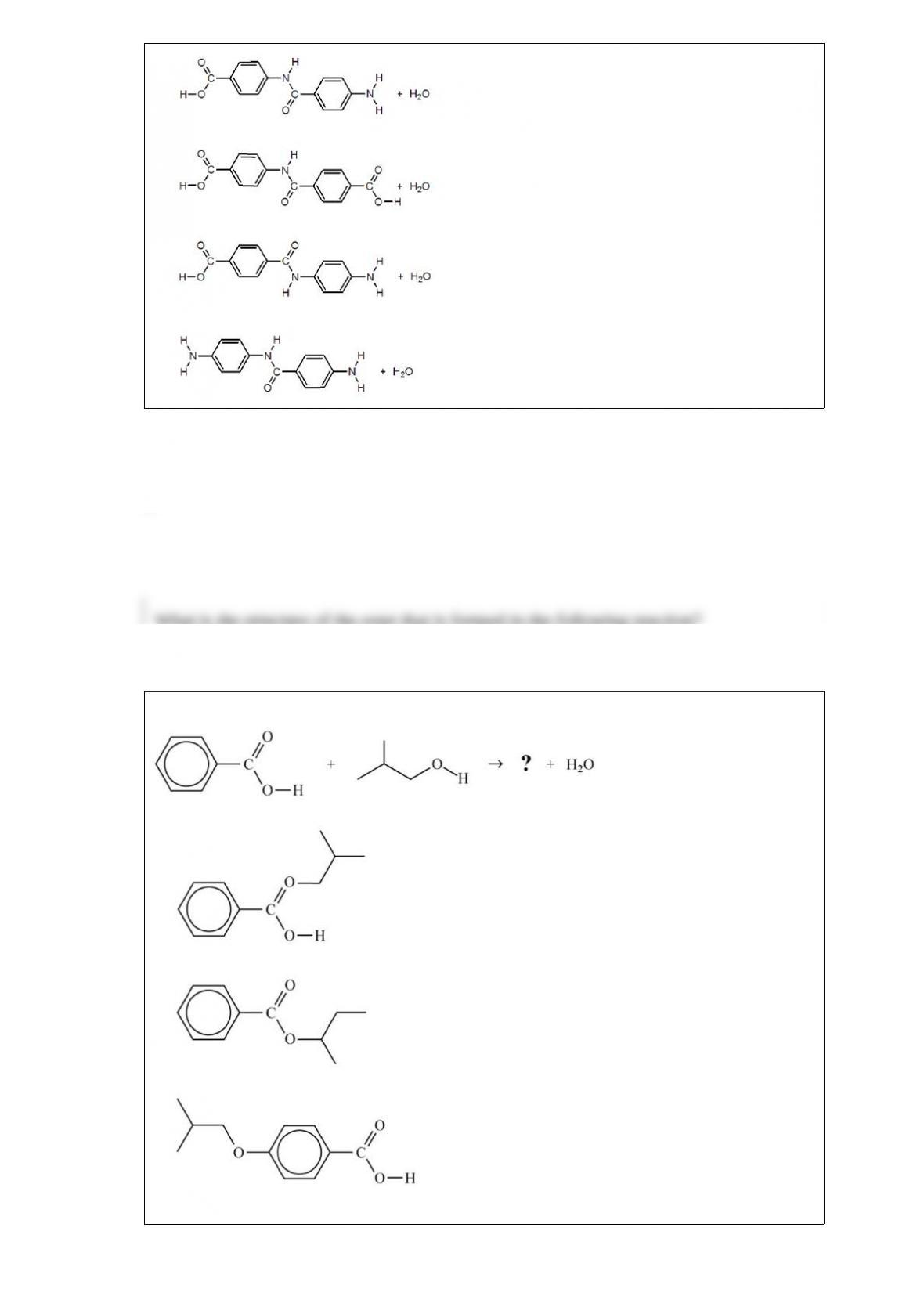
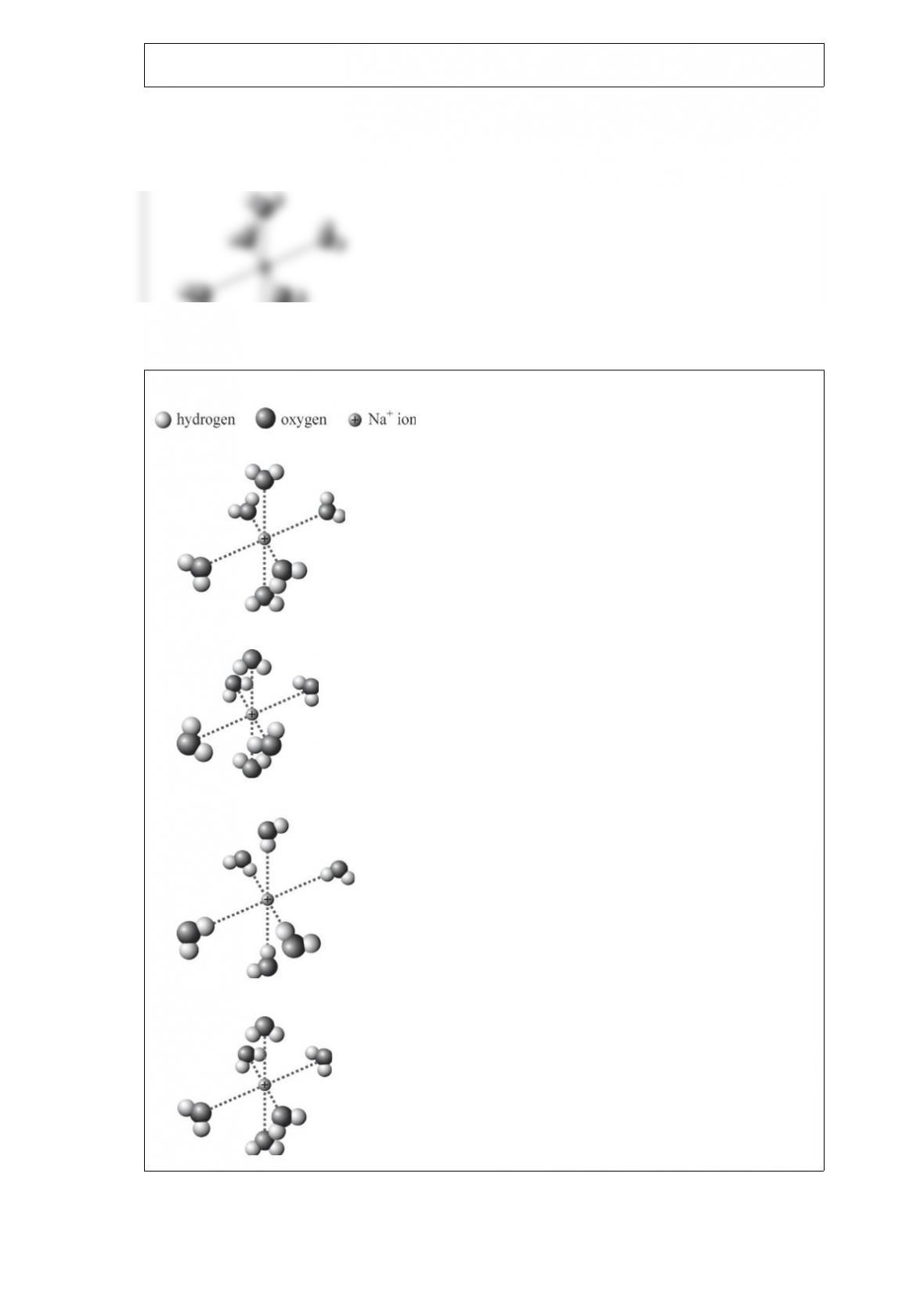
A. 0.369
B. 3.69
C. 36.9
D. 369
The goal of the Montreal Protocol in 1987 was to
A. reduce the amount of new production of chlorofluorocarbons in developed countries.
B. recycle existing chlorofluorocarbons rather than release them into the air.
C. encourage research into substitutes for chlorofluorocarbons.
D. All of these choices are correct.
In their fourth and most recent report published in 2007, the vast majority of scientists
agreed on several key points. Which of the following is NOT one of those points?
A. If the rate of greenhouse gas emissions is not curtailed, our water resources, food
supply, and even our health will suffer.
B. Human activities (primarily the combustion of fossil fuels and deforestation) are
responsible for much of the recent warming.