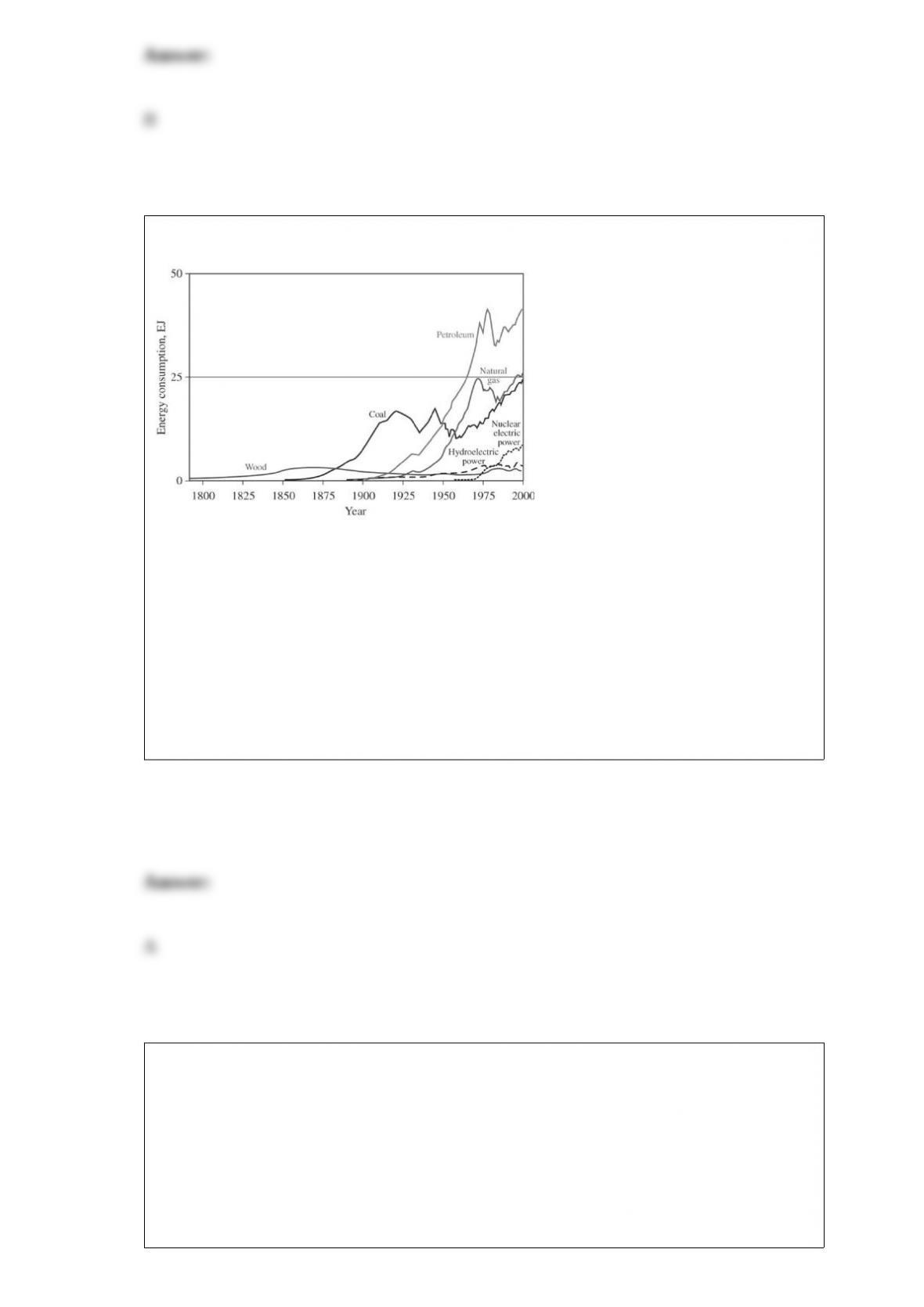
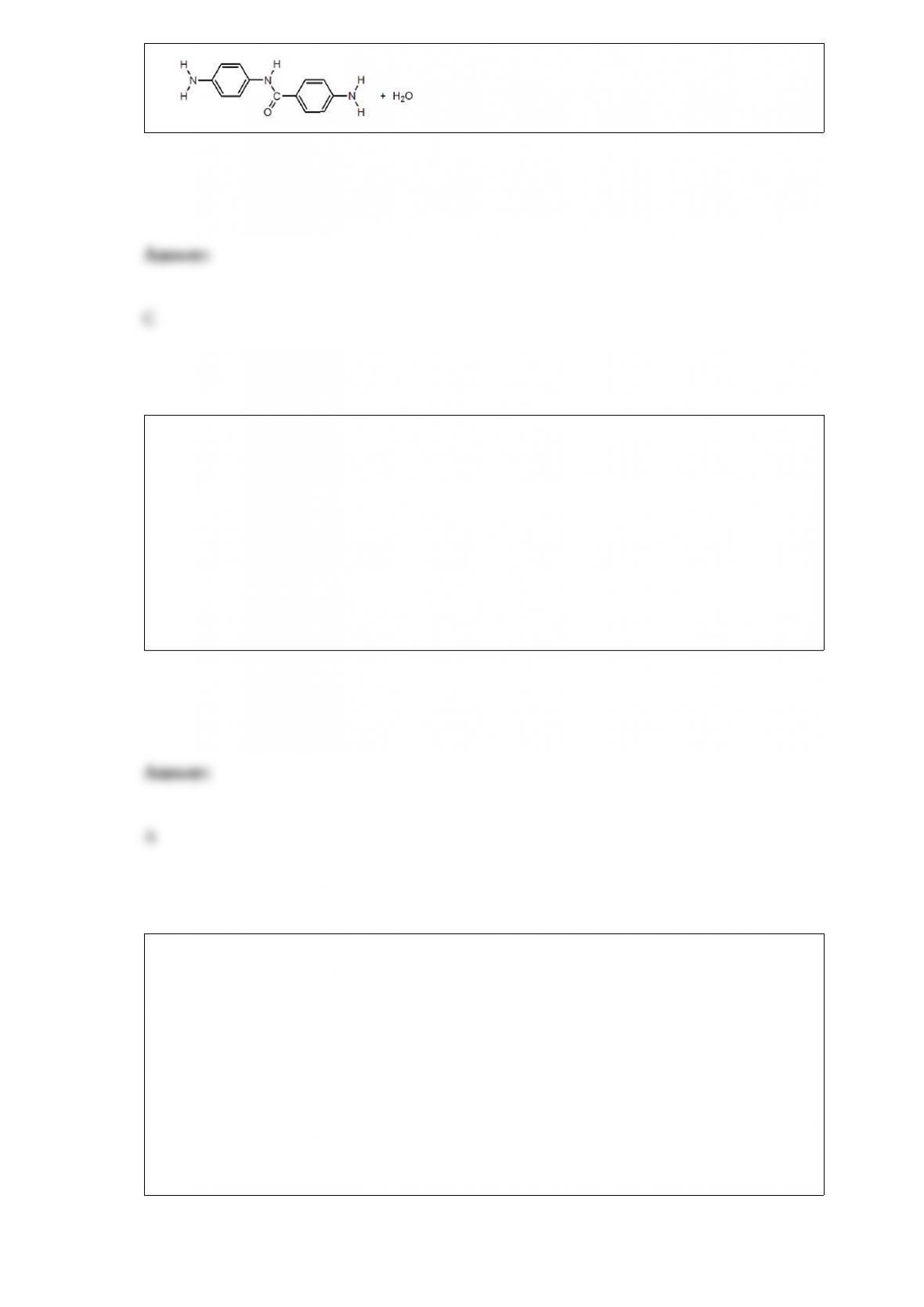
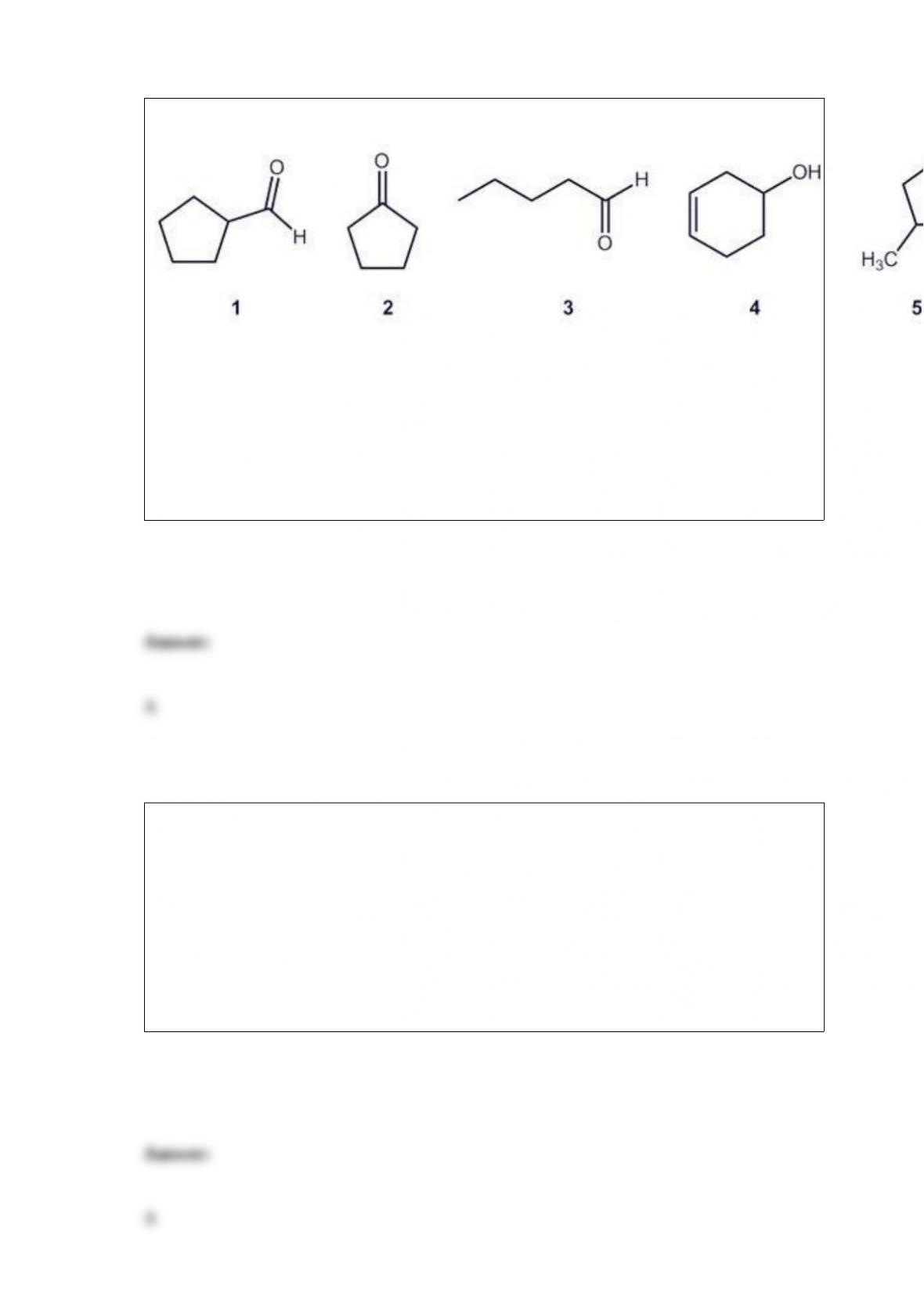
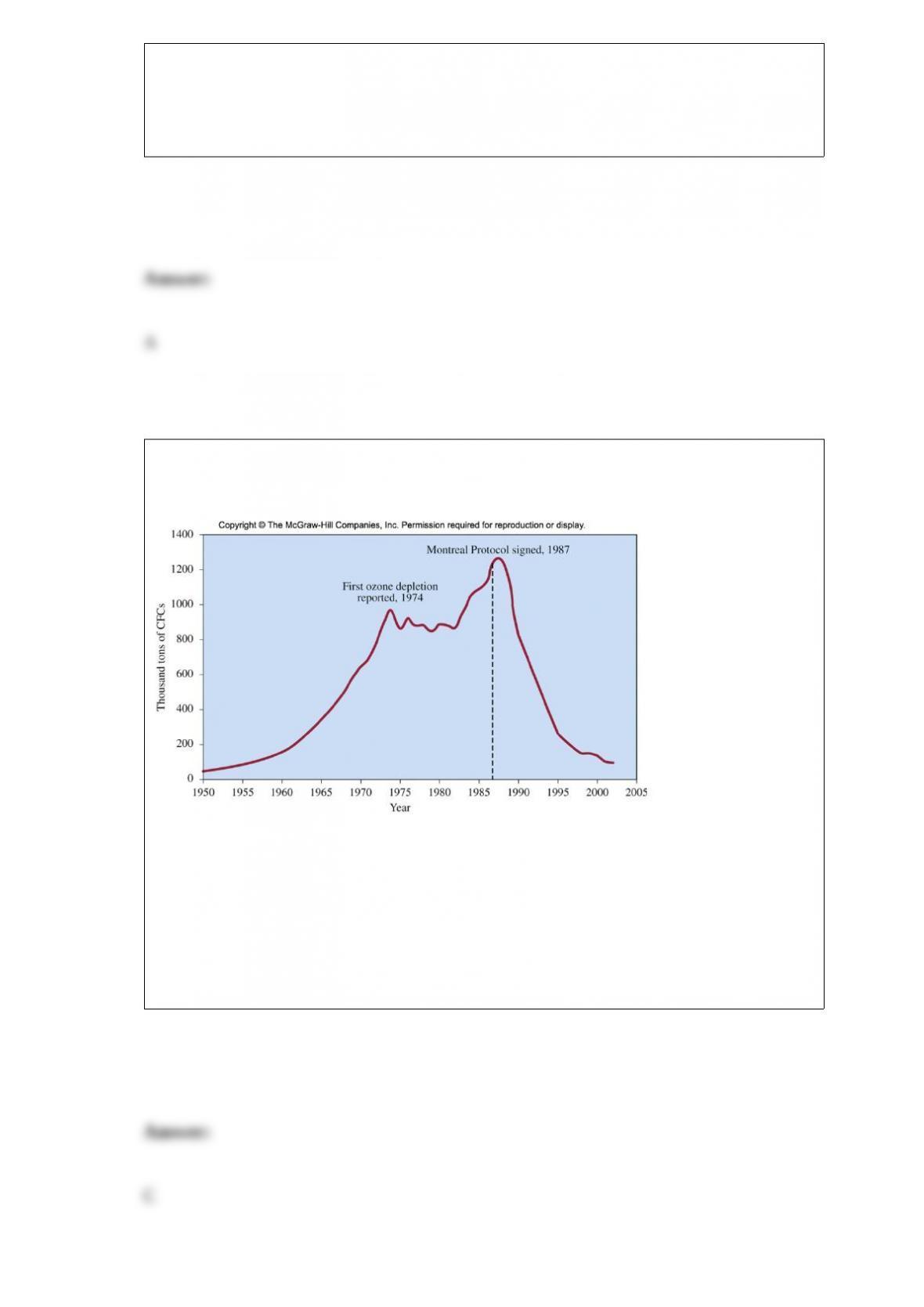
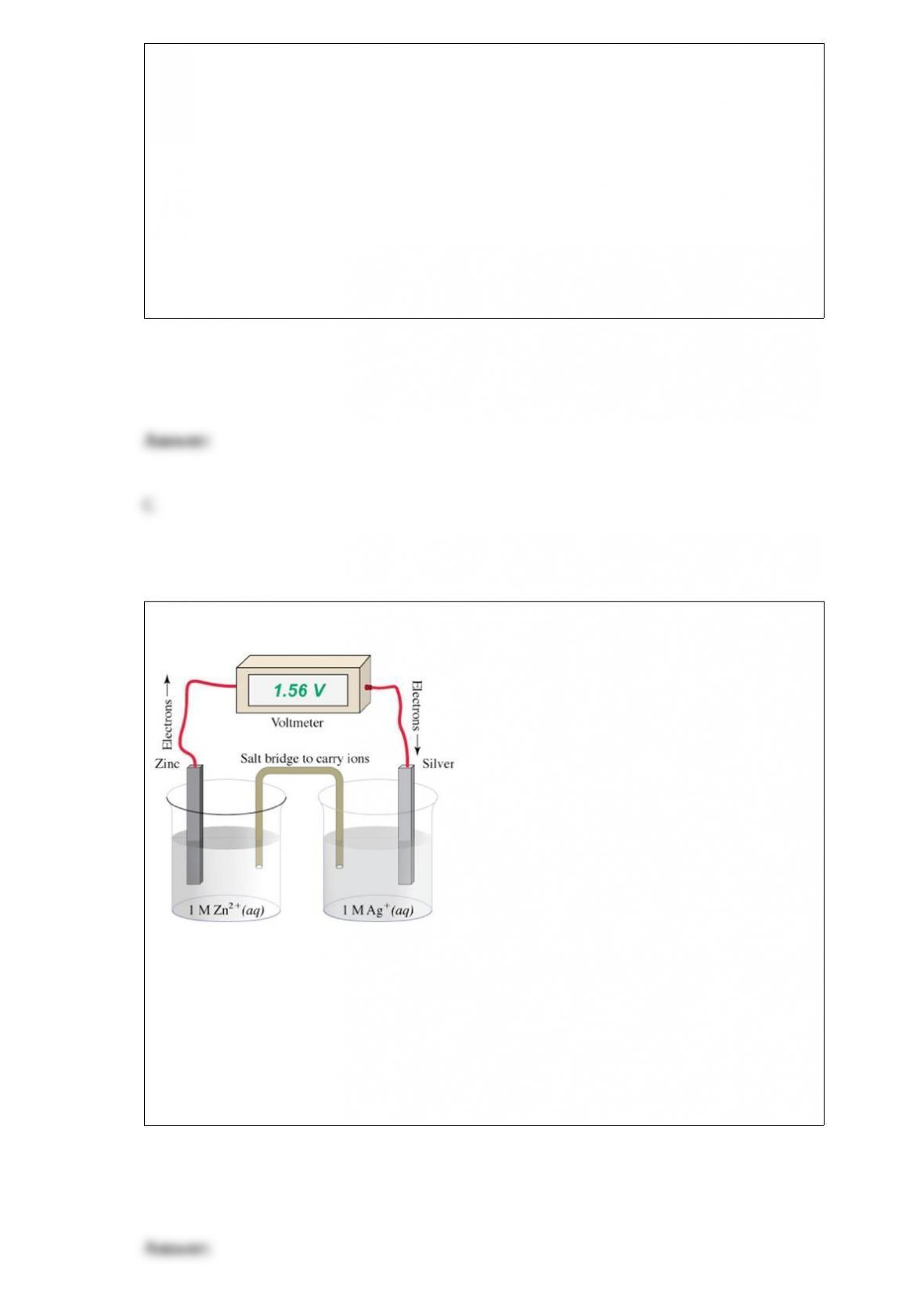
B. Absorb hydrogen onto activated charcoal; then heat the mixture to release the
hydrogen.
C. Store it in the form of ionic metal hydrides, such as LiH, which release hydrogen gas
when they react with water.
D. Encapsulate hydrogen molecules in fullerene molecules (large, carbon-based
molecules that can act like cages) that may be heated later to release the hydrogen.
Batteries must be used in addition to solar cells when generating household electricity
because
A. solar cells can generate electricity only via the output of a battery.
B. solar cells can generate only a small fraction of the total energy needed by a
household at any one time.
C. solar cells generate so much electricity that they will overheat if they cannot transfer
the excess electricity somewhere to dissipate the extra heat.
D. batteries must store and then supply the energy when sunlight is not available.
Which would be classified as a "natural" polymer?
A. cellulose
B. polystyrene