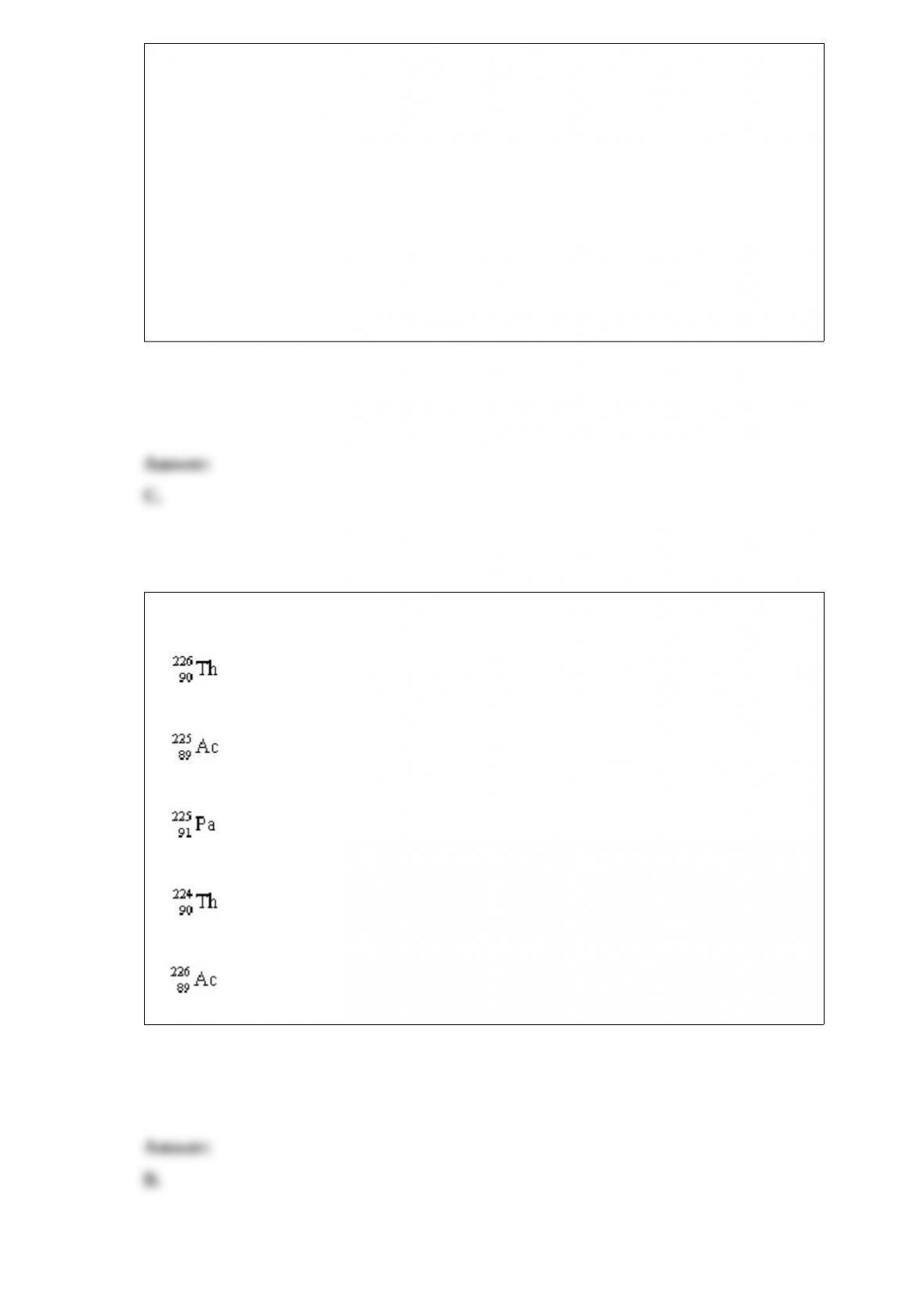
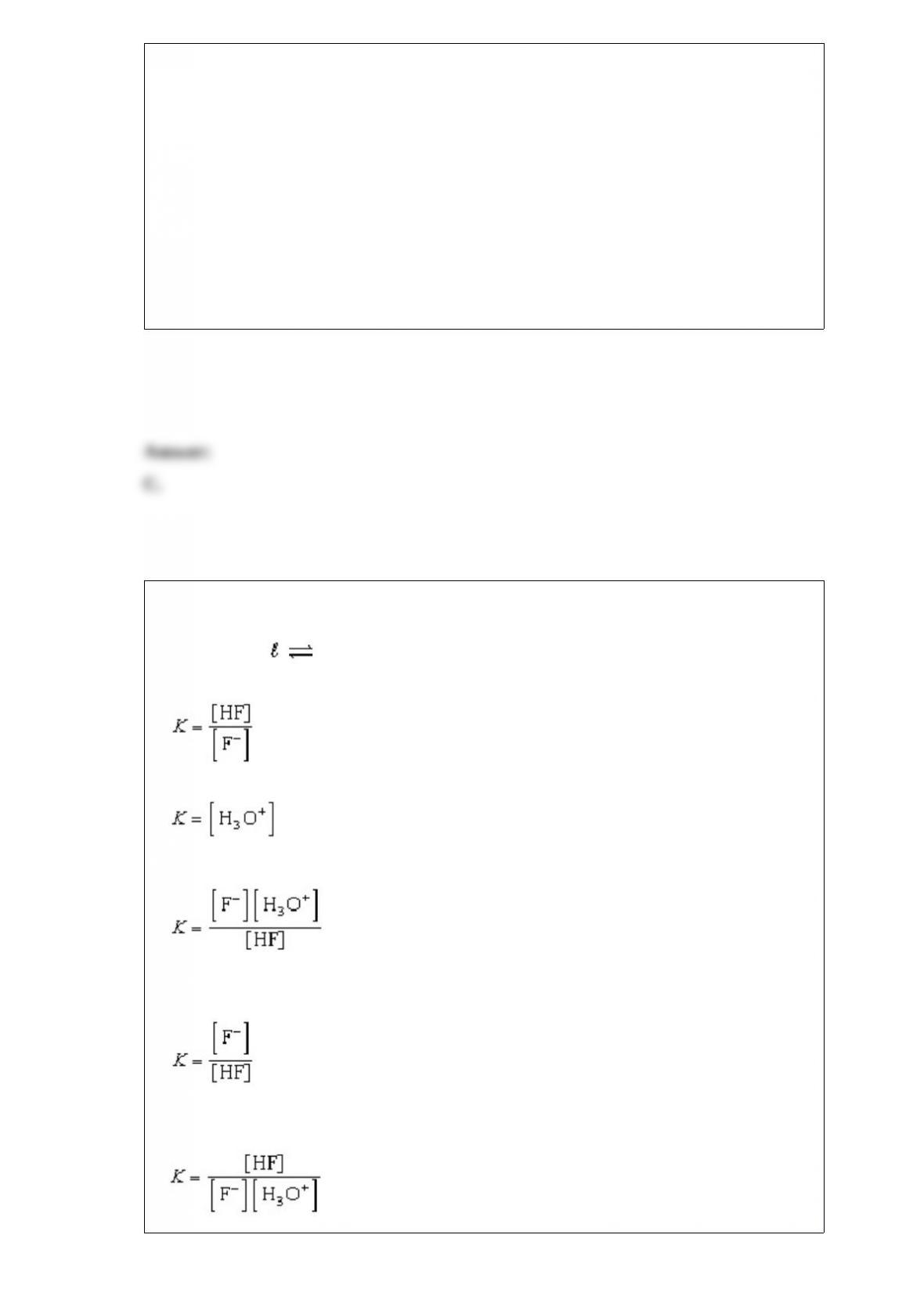
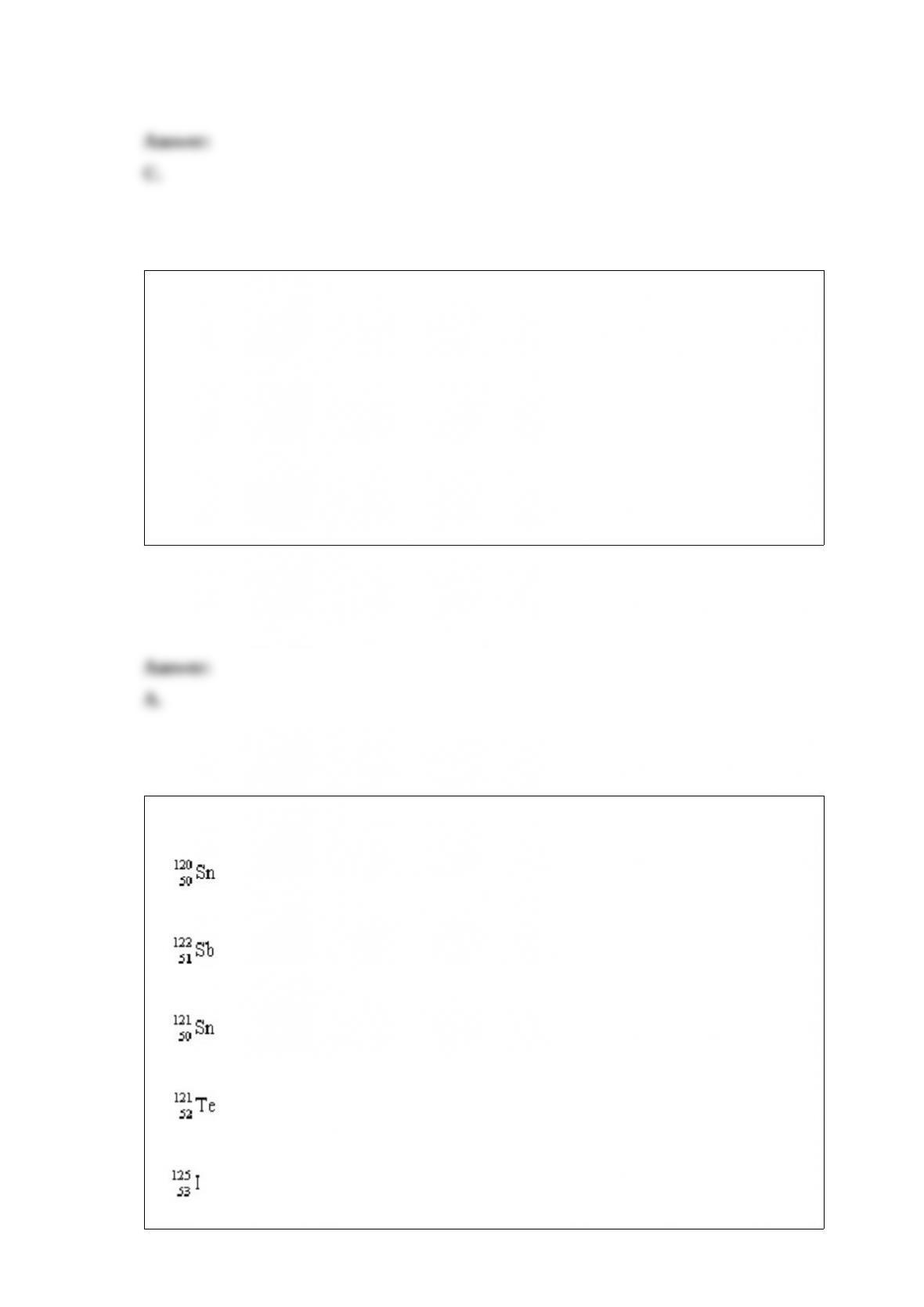
What are the possible geometries of a metal complex with a coordination number of 4?
1/ square planar
2/ tetrahedral
3/ octahedral
A.1 only
B.2 only
C.3 only
D.1 and 2
E.1, 2, and 3
The ____ of a photon of light is ____ proportional to its frequency and ____
proportional to its wavelength.
A.energy, directly, inversely
B.energy, inversely, directly
C.velocity, directly, inversely
D.intensity, inversely, directly
E.amplitude, directly, inversely