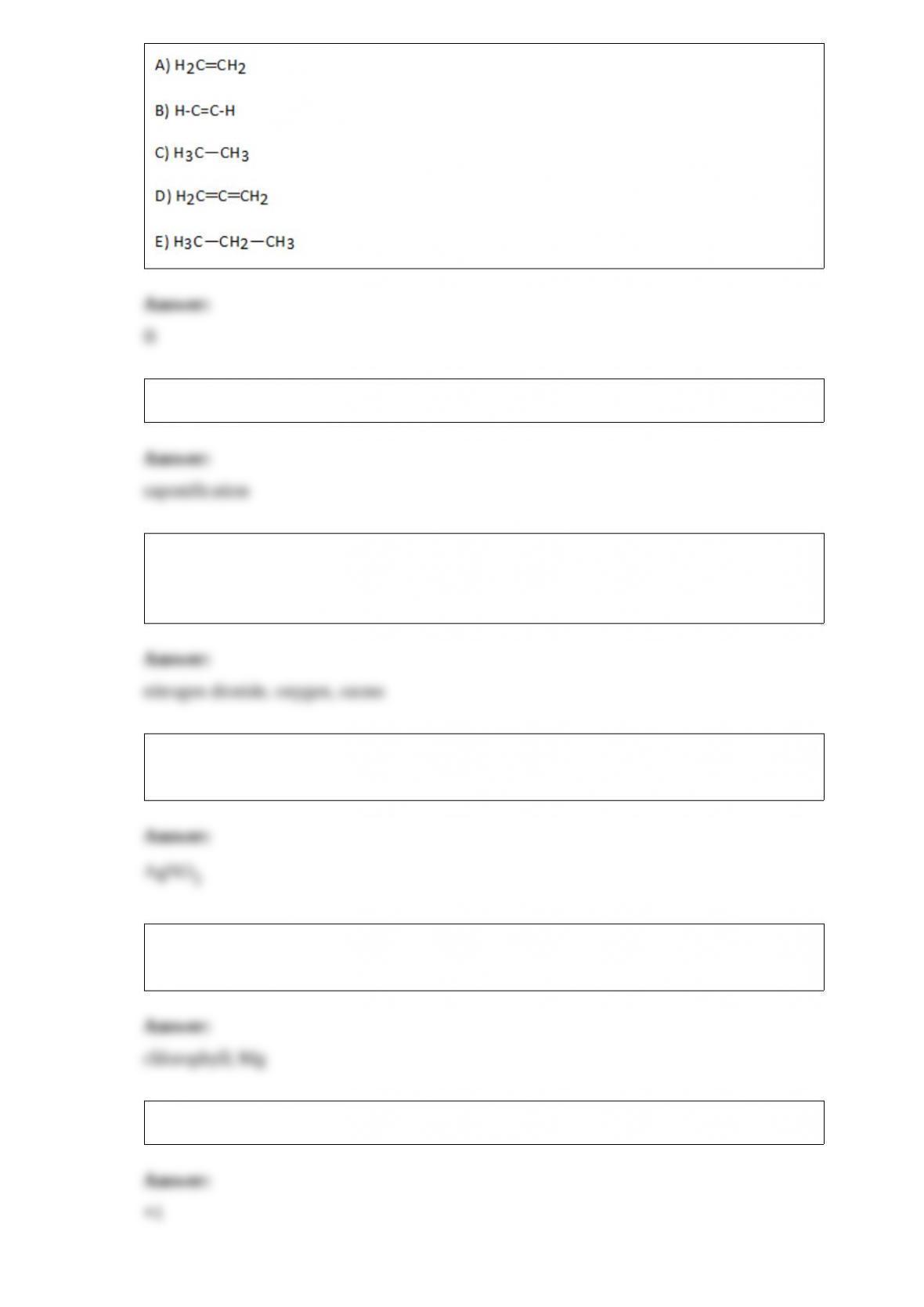
1) Coordination sphere isomers ________.
A) have the same molecular formula and coordination number
B) have the same molecular formula but different coordination numbers
C) have different molecular formulas but the same coordination number
D) have different molecular formulas and different coordination numbers
E) are the same as resonance structures
2) The elementary reaction
2NO2 (g) --> 2NO (g) + O2 (g)
is second order in NO2 and the rate constant at 660 K is 5.23 M-1s-1. The reaction
half-life at this temperature when [NO2]0 = 0.45 M is ________ s.
A) 2.4
B) 7.6
C) 0.19
D) 0.13
E) 0.42
3) What is the predominant intermolecular force in HCN?
A) dipole-dipole attraction
B) ion-dipole attraction
C) ionic bonding
D) hydrogen bonding
E) London dispersion forces
4) In the nuclear transmutation represented by Pu( He, n)?, what is the product?
A) uranium-242
B) curium-245
C) curium-242
D) uranium-245
E) uranium-243