B) 100 g of the solution contains 15 g of benzene
C) 1.0 g of the solution contains 15 x 10-6 g of benzene
D) 1.0 L of the solution contains 15 g of benzene
E) the solution is 15% by mass of benzene
14) S is negative for the reaction ________.
A) 2SO2 (g) + O2 (g) --> 2SO3 (g)
B) NH4Cl (s) --> NH3 (g) + HCl (g)
C) PbCl2 (s) --> Pb2+ (aq) + 2Cl- (aq)
D) 2C (s) + O2 (g) --> 2CO2 (g)
E) H2O (l) --> H2O (g)
15) Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose
(molar mass = 180.2 g/mol) in 920.0 ml of water at 25 oC. The vapor pressure of pure
water at 25 oC is 23.76 mm Hg. Assume the density of the solution is 1.00 g/ml.
A) 0.278 mm Hg
B) 0.605 mm Hg
C) 22.98 mm Hg
D) 23.48 mm Hg
E) 23.76 mm Hg
16) Of the following, which is the weakest acid?
A) HPO3
-
B) H3PO4
C) H2PO4
-
D) HPO4
-
E) The acid strength of all of the above is the same.
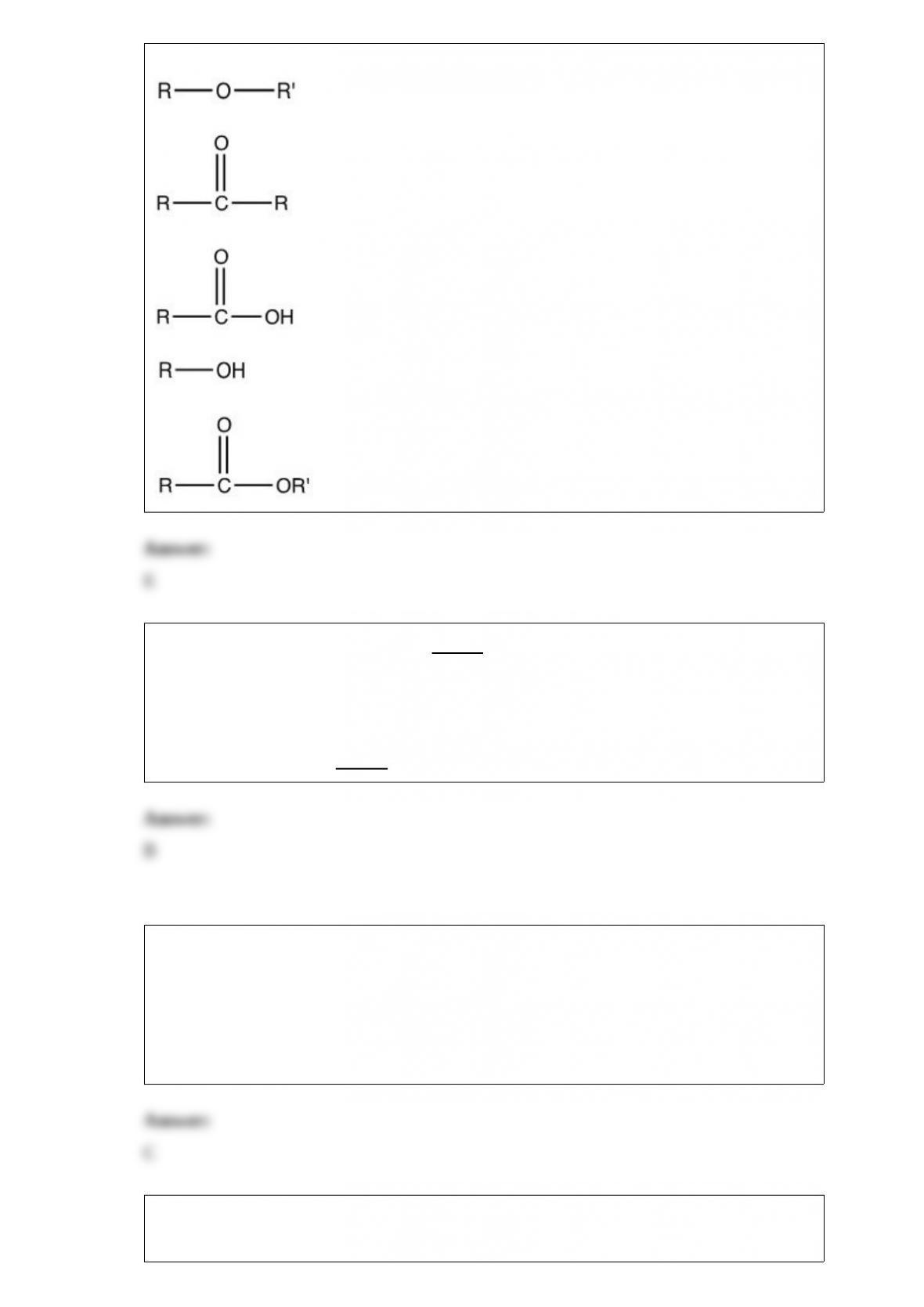
17) The general formula of an ester is ________.