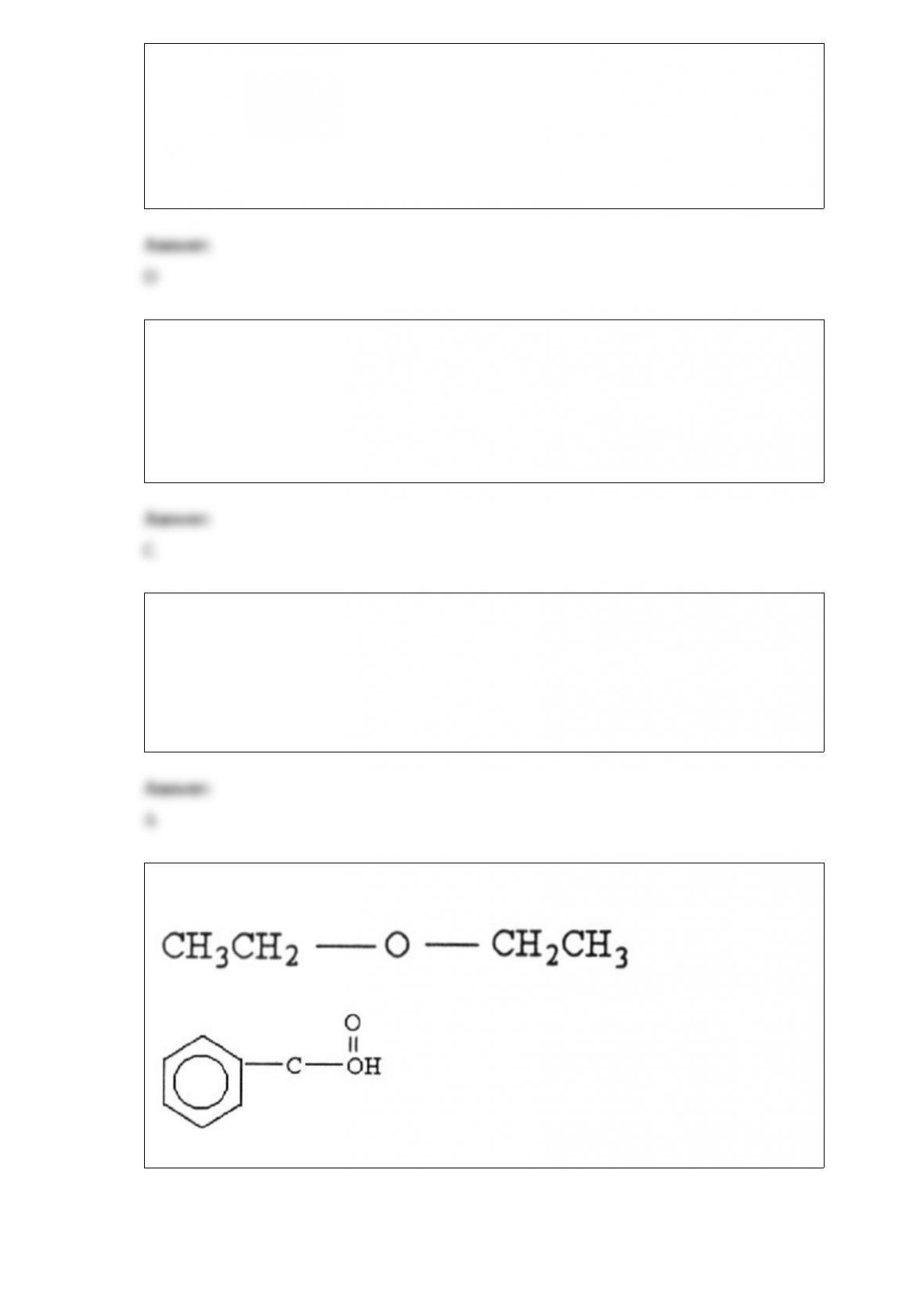
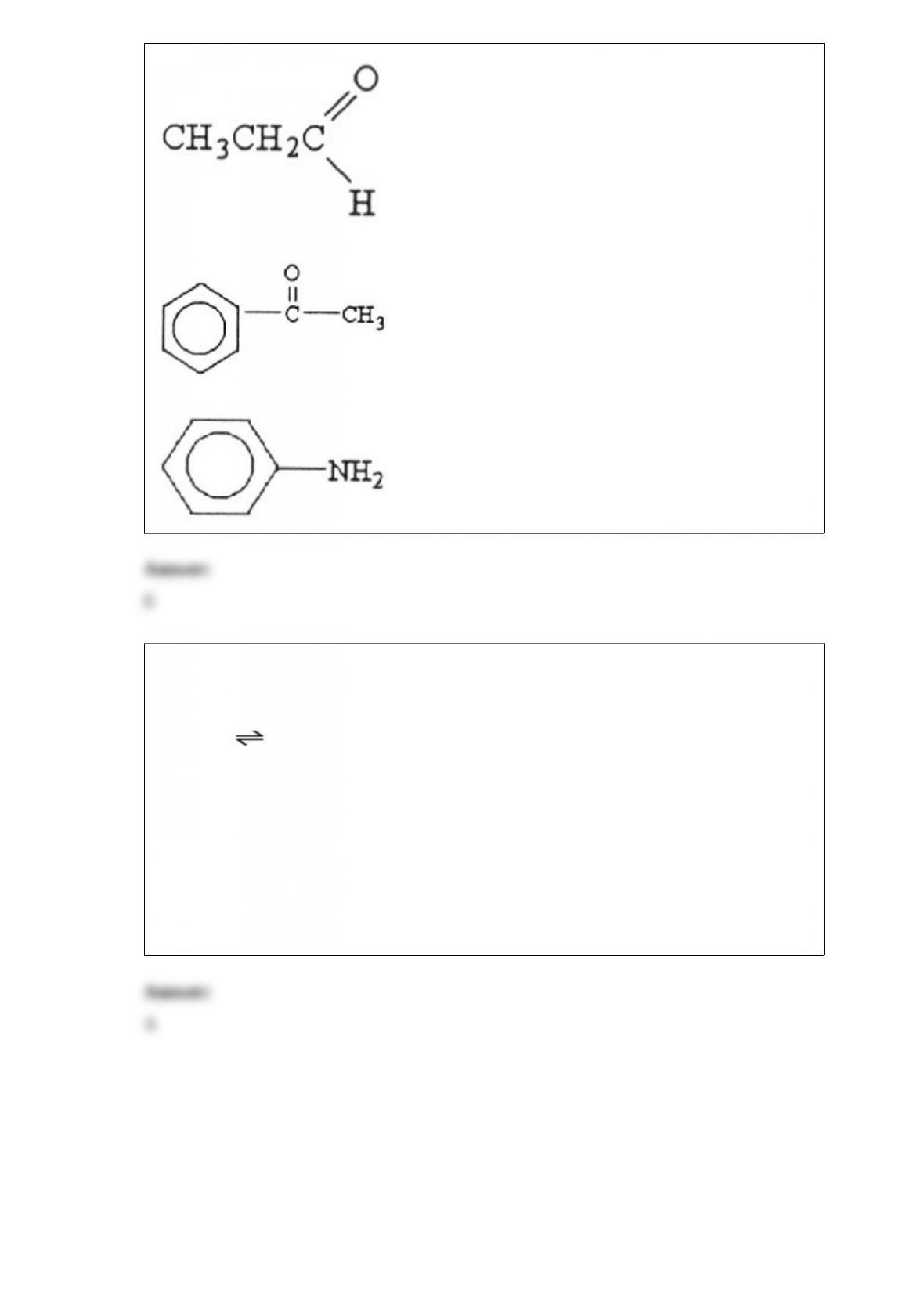
5) Round the number 0.00637 to two significant figures.
A) 0.0064
B) 0.006
C) 0.00637
D) 0.0
E) 0.0063700
6) ________ is a polysaccharide.
A) Cellulose
B) Galactose
C) Ribose
D) Sucrose
E) Uracil
7) What is the final stage in municipal water treatment?
A) treatment with ozone or chlorine
B) course filtration through a screen
C) addition of CaO/Al2(SO4)3 and settling
D) sand bed filtration
E) aeration
8) Which of the following statements is false?
A) Internal energy is a state function.
B) Enthalpy is an intensive property.
C) The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to the
enthalpy change for the reverse reaction.
D) The enthalpy change for a reaction depends on the state of the reactants and
products.
E) The enthalpy of a reaction is equal to the heat of the reaction.