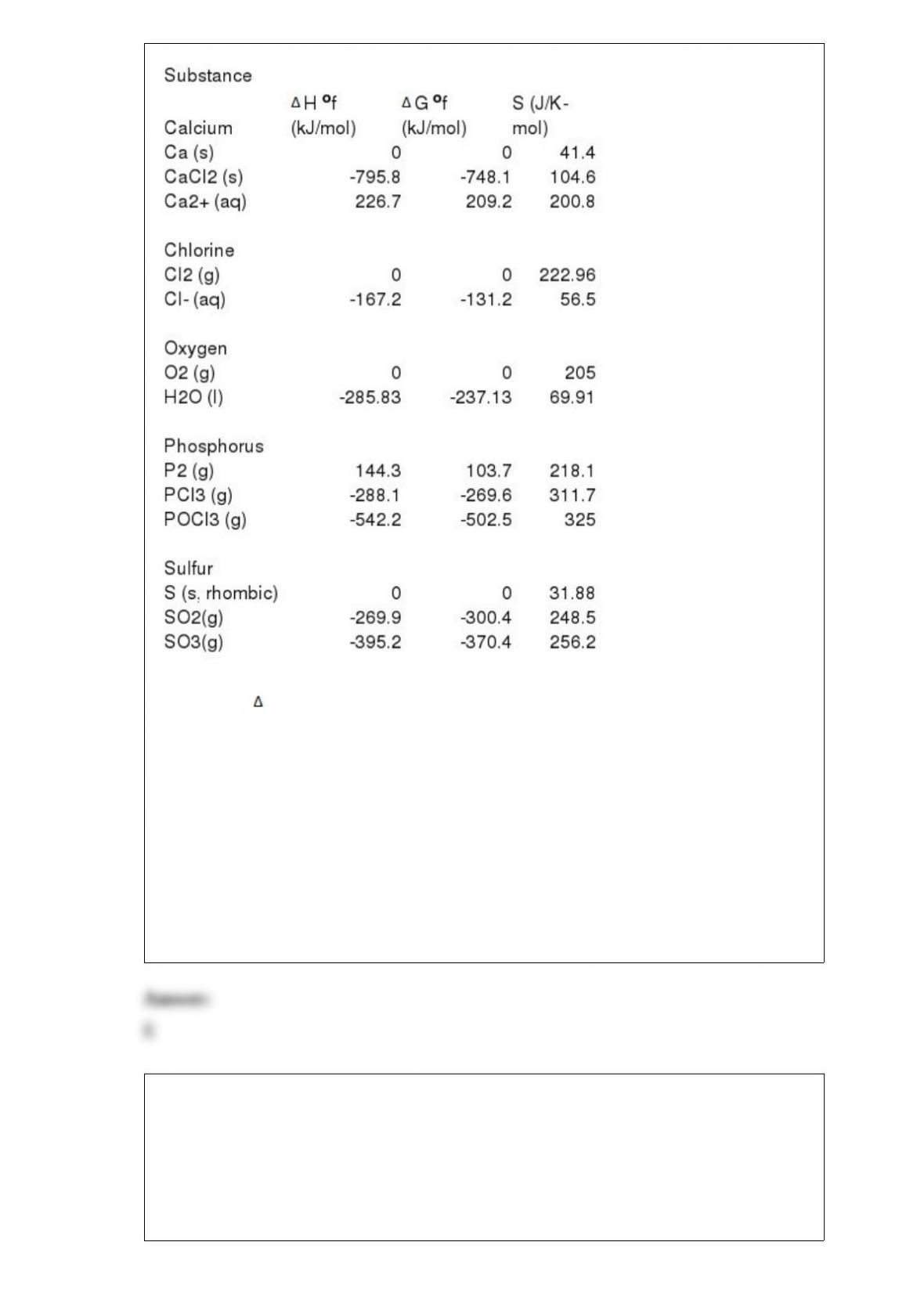
1) Solids have a ________ shape and are not appreciably ________.
A) definite, compressible
B) definite, incompressible
C) indefinite, compressible
D) indefinite, incompressible
E) sharp, convertible
2) A reaction that is not spontaneous at low temperature can become spontaneous at
high temperature if H is ________ and S is ________.
A) +, +
B) -, -
C) +, -
D) -, +
E) +, 0
3) The correct name for MgF2 is ________.
A) monomagnesium difluoride
B) magnesium difluoride
C) manganese difluoride
D) manganese bifluoride
E) magnesium fluoride
4) The phrase "like dissolves like" refers to the fact that ________.
A) gases can only dissolve other gases
B) polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
C) solvents can only dissolve solutes of similar molar mass
D) condensed phases can only dissolve other condensed phases
E) polar solvents dissolve nonpolar solutes and vice versa
5) S is negative for the reaction ________.
A) Sr(NO3)2 (aq) + 2LiOH (aq) --> Sr(OH)2 (s) + 2LiNO3 (aq)
B) 2H2O (g) --> 2H2 (g) + O2 (g)