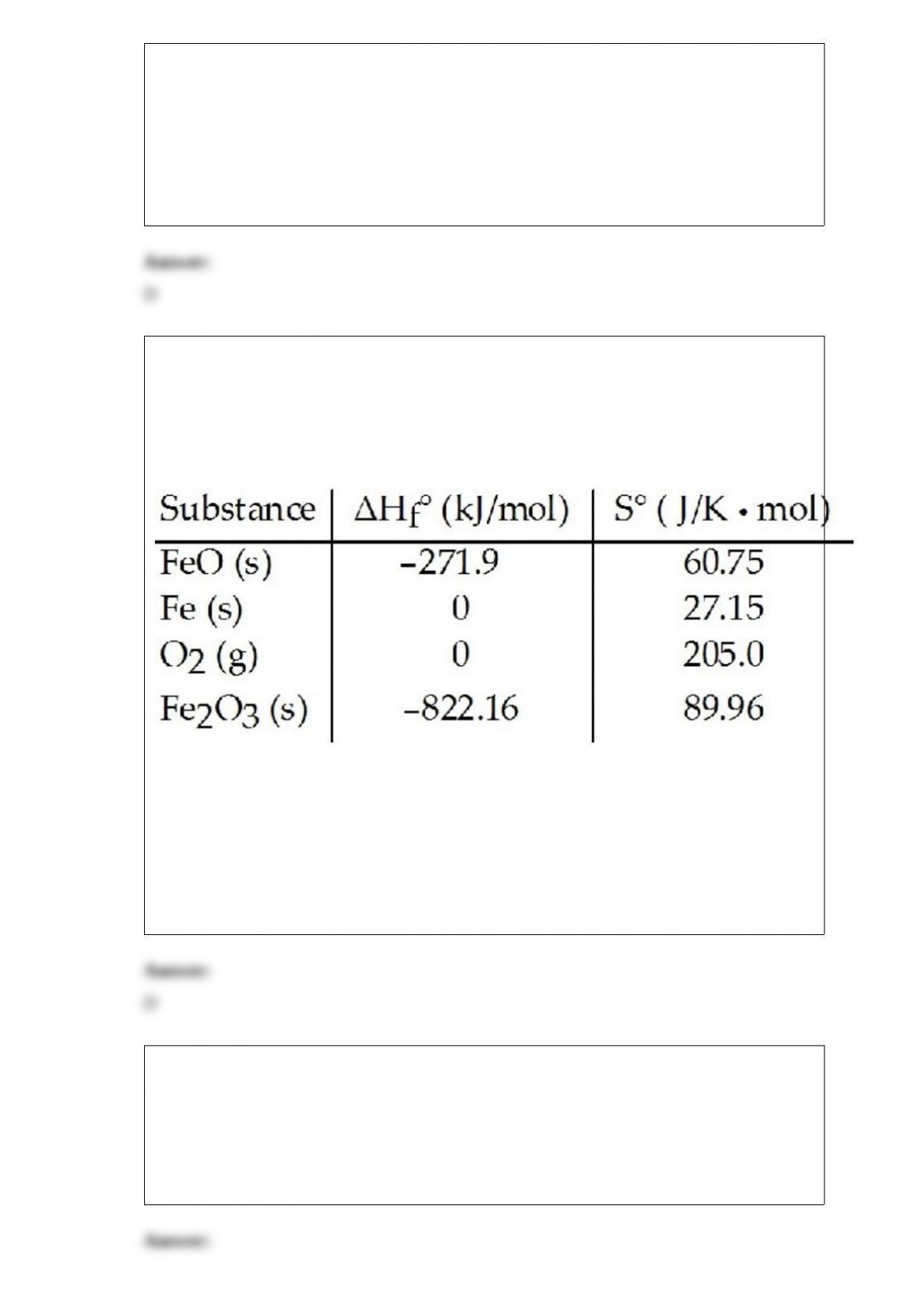
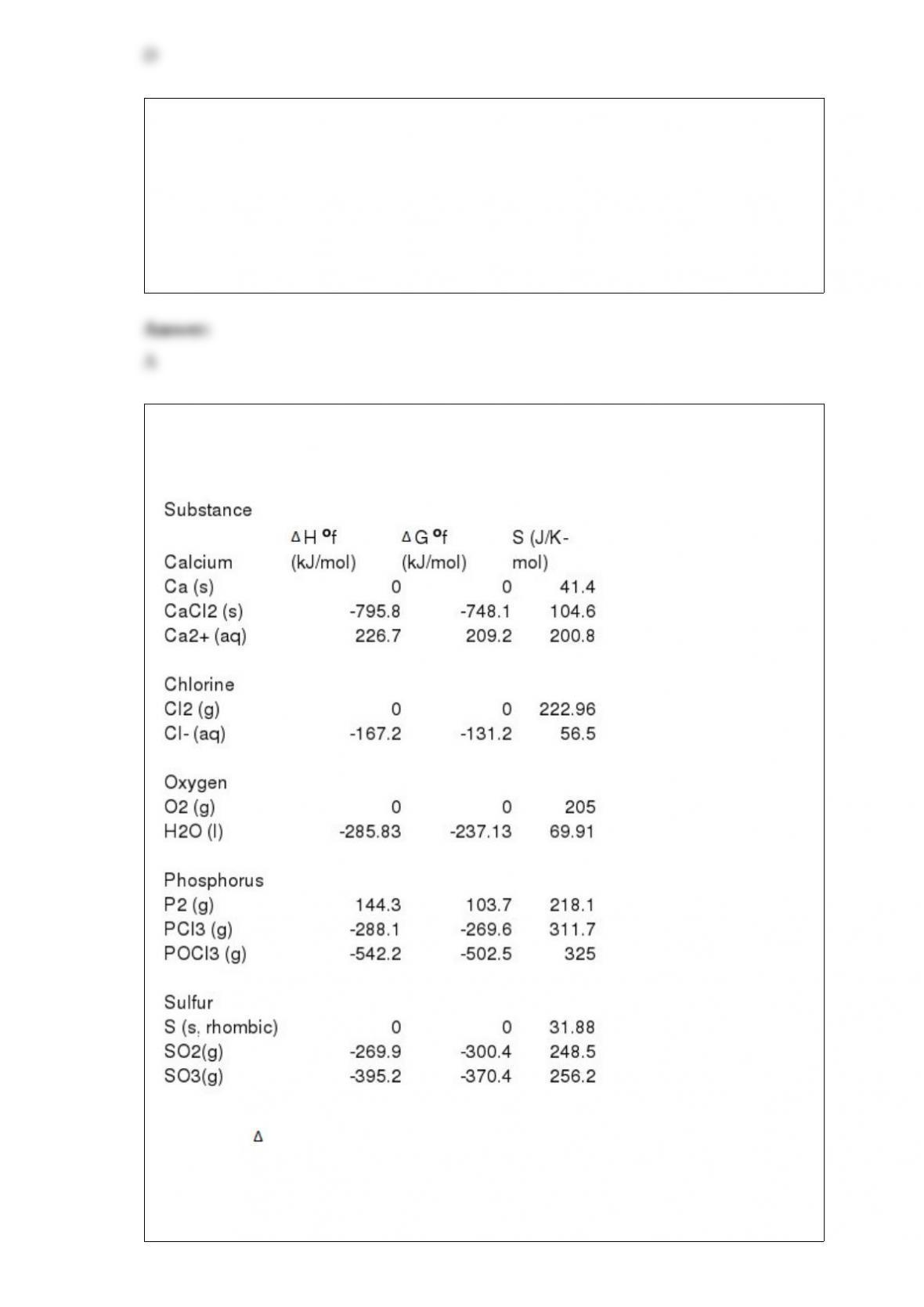
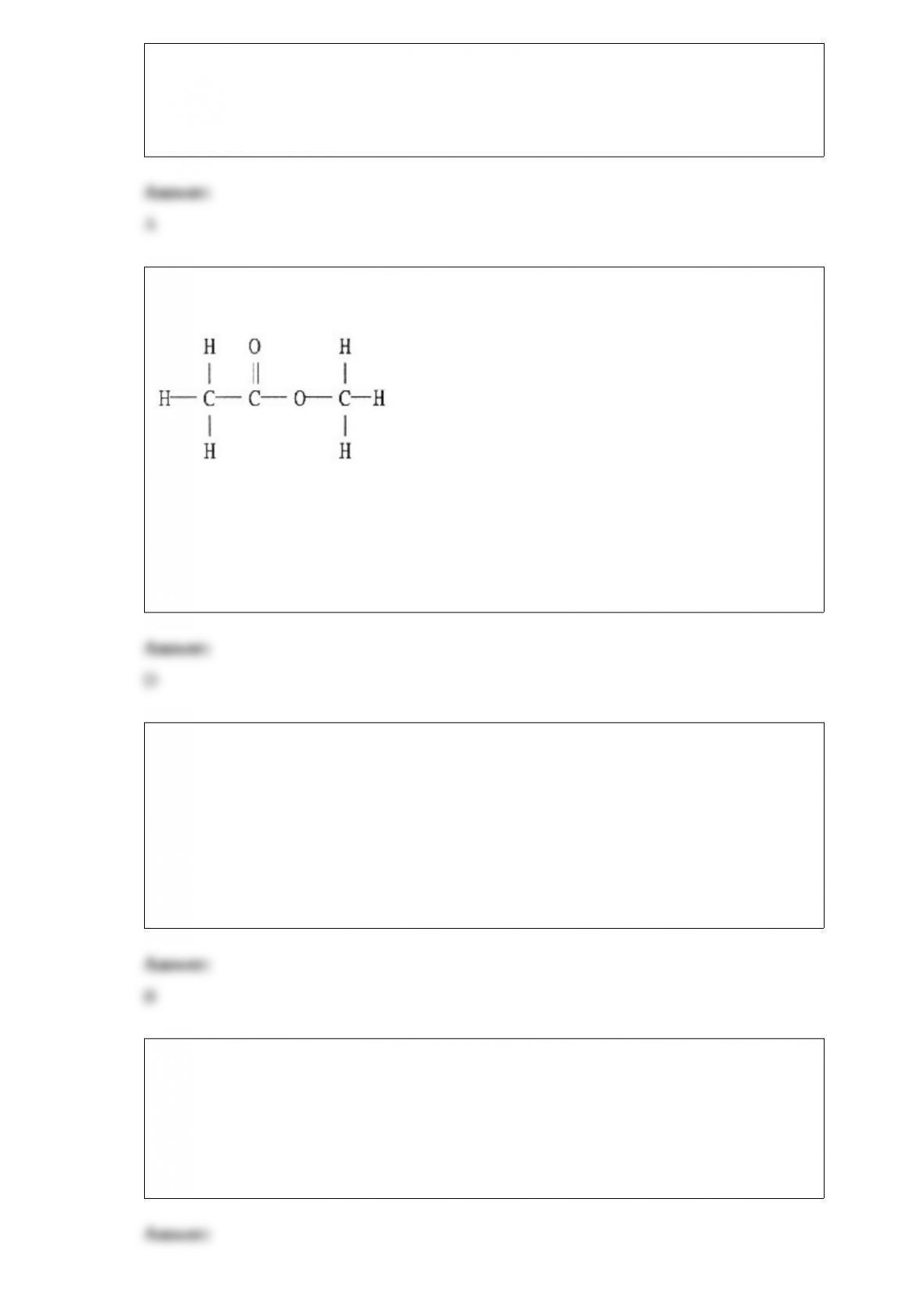
17) What is the typical percent of uranium-235 in the enriched UO2 pellets used in
nuclear reactors?
A) 0.7
B) 1
C) 3
D) 10
E) 14
18) Which one of the following is not true about transition metals?
A) They frequently have more than one common oxidation state.
B) Their compounds are frequently colored.
C) Their compounds frequently exhibit magnetic properties.
D) They are found in the d-block of the periodic table.
E) They typically have low melting points.
19) Given the equation
H2O (l) → H2O (g) ΔHrxn = 40.7 kJ at 100 °C
Calculate the heat required to convert 3.00 grams of liquid water at 100 °C to vapor.
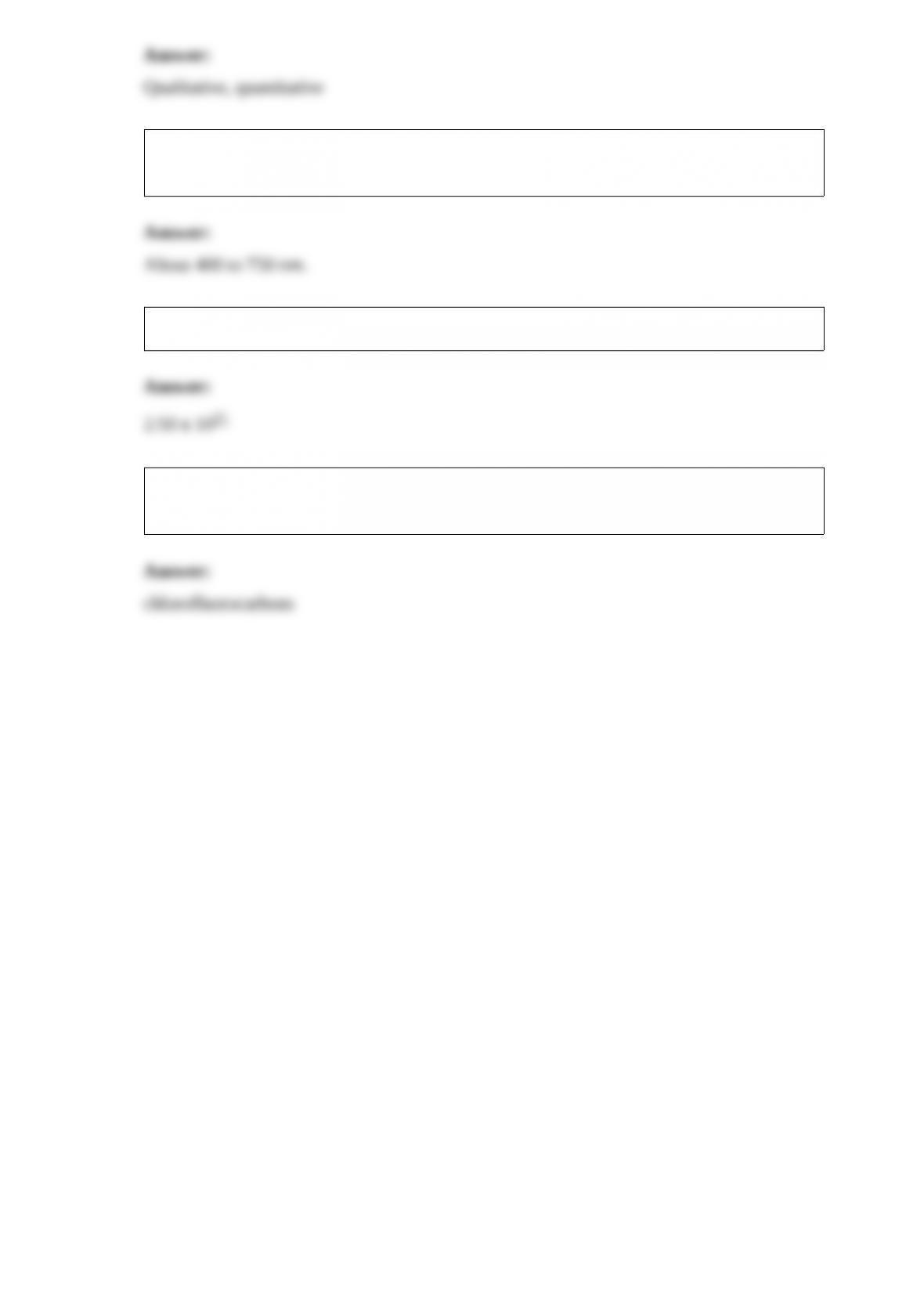
20) What are the principal components used in making soda-lime glass?
21) All of the group VIA elements are solids except ________.
22) ________ analysis determines only the presence or absence of a particular metal
ion, whereas ________ analysis determines how much of a given substance is present.