5) Which one of the following is false concerning buckminsterfullerene?
A) It is the most recently discovered crystalline allotrope of carbon.
B) It consists of individual molecules like C60and C70.
C) It is a molecular form of carbon.
D) It is made up of Cl2 molecules.
E) It is made up of molecules that resemble soccer balls.
6) Calculate the value of ΔE in joules for a system that loses 115 J of heat and has 150 J
of work performed on it by the surroundings.
A) -115 J
B) -35 J
C) +35 J
D) +265 J
E) -265 J
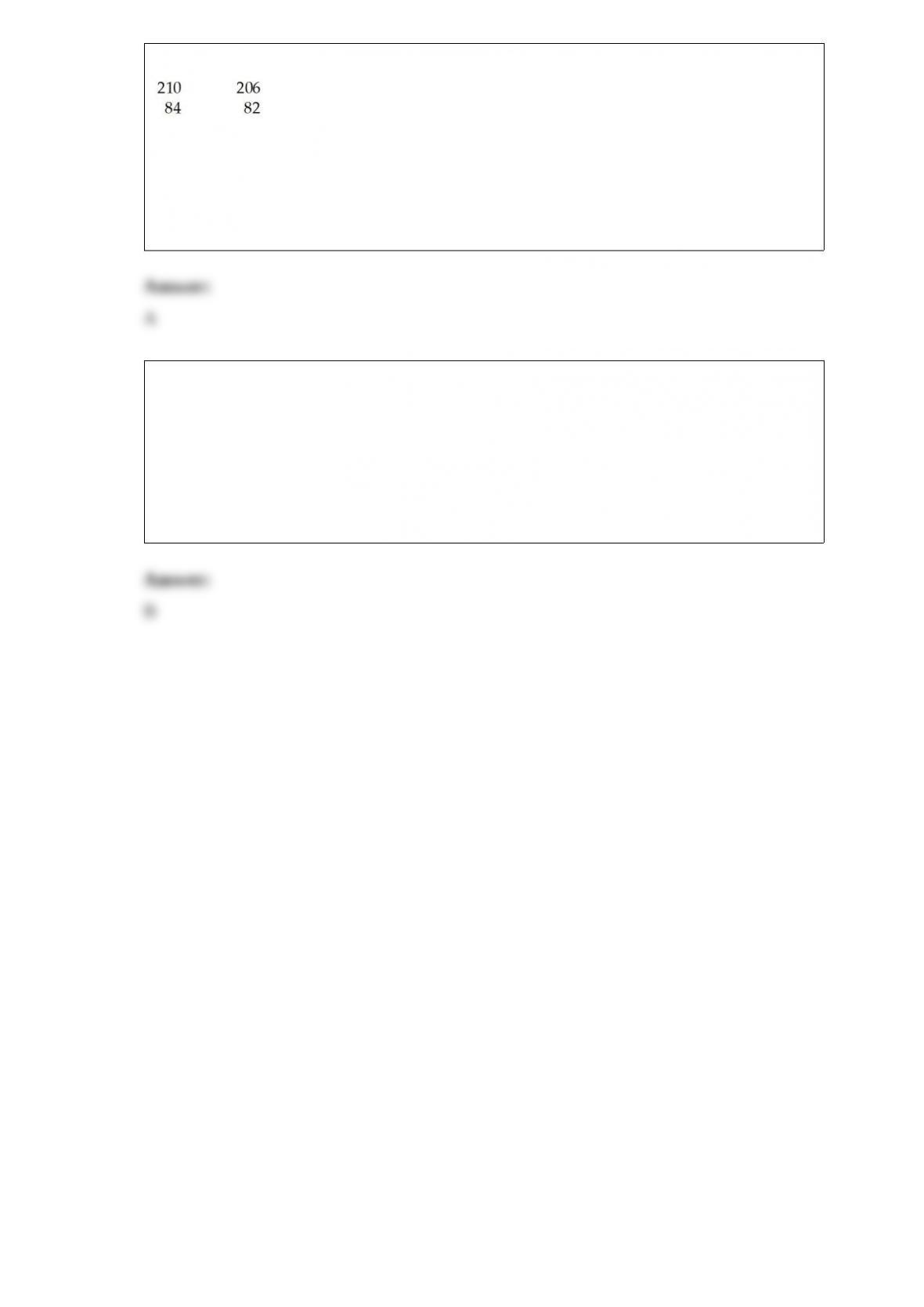
7) The pH of a solution prepared by dissolving 0.350 mol of solid methylamine
hydrochloride (CH3NH3Cl) in 1.00 L of 1.10 M methylamine (CH3NH2) is ________.
The Kb for methylamine is
4.40 x 10-4. (Assume the final volume is 1.00 L.)
A) 1.66
B) 2.86
C) 10.28
D) 11.14
E) 10.61
8) The kinetic energy of a 10.3 g golf ball traveling at 48.0 m/s is ________ J.
A) 1.20 x 103
B) 66
C) 11.9
D) 1.3 x 102
E) 23.7