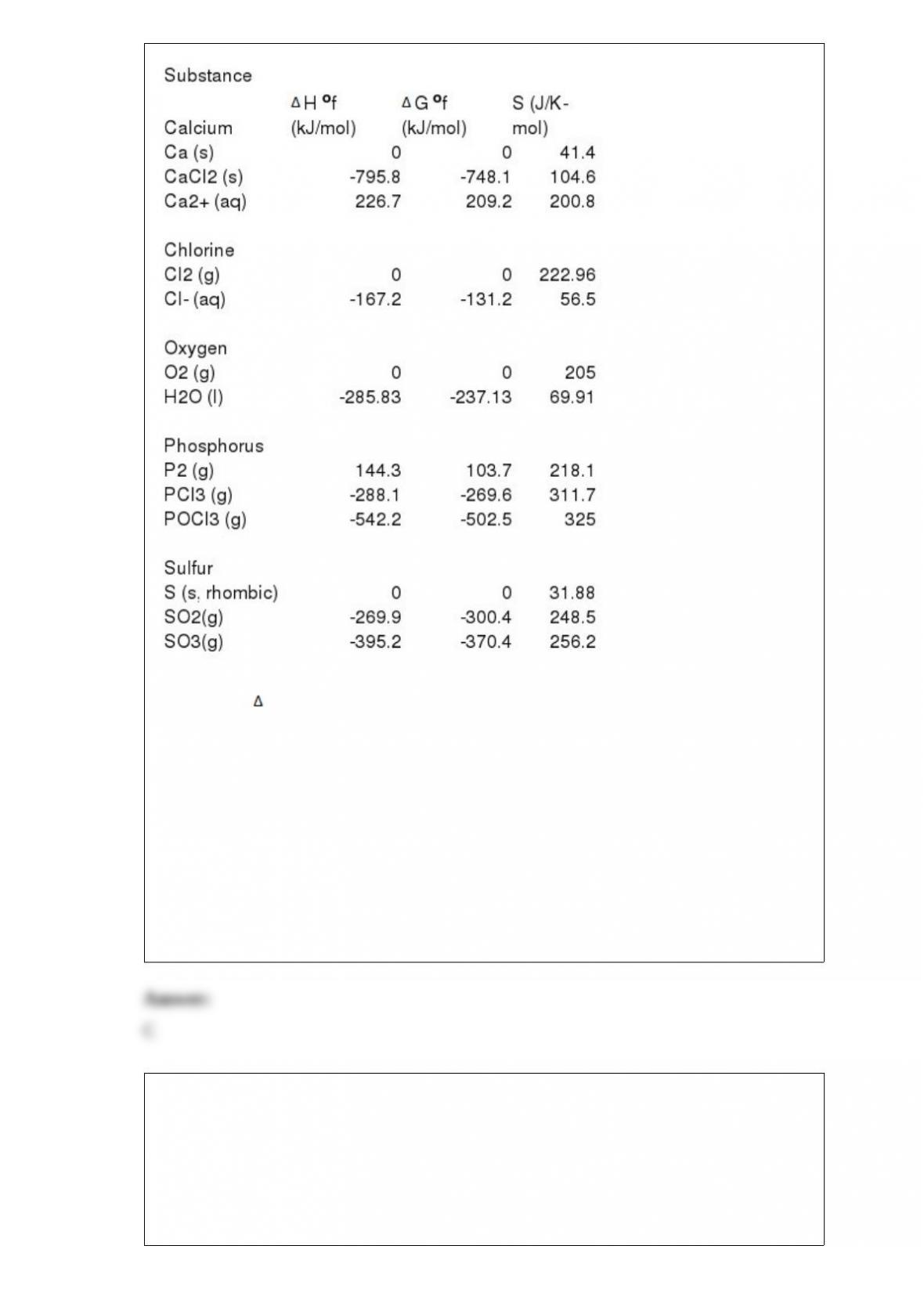
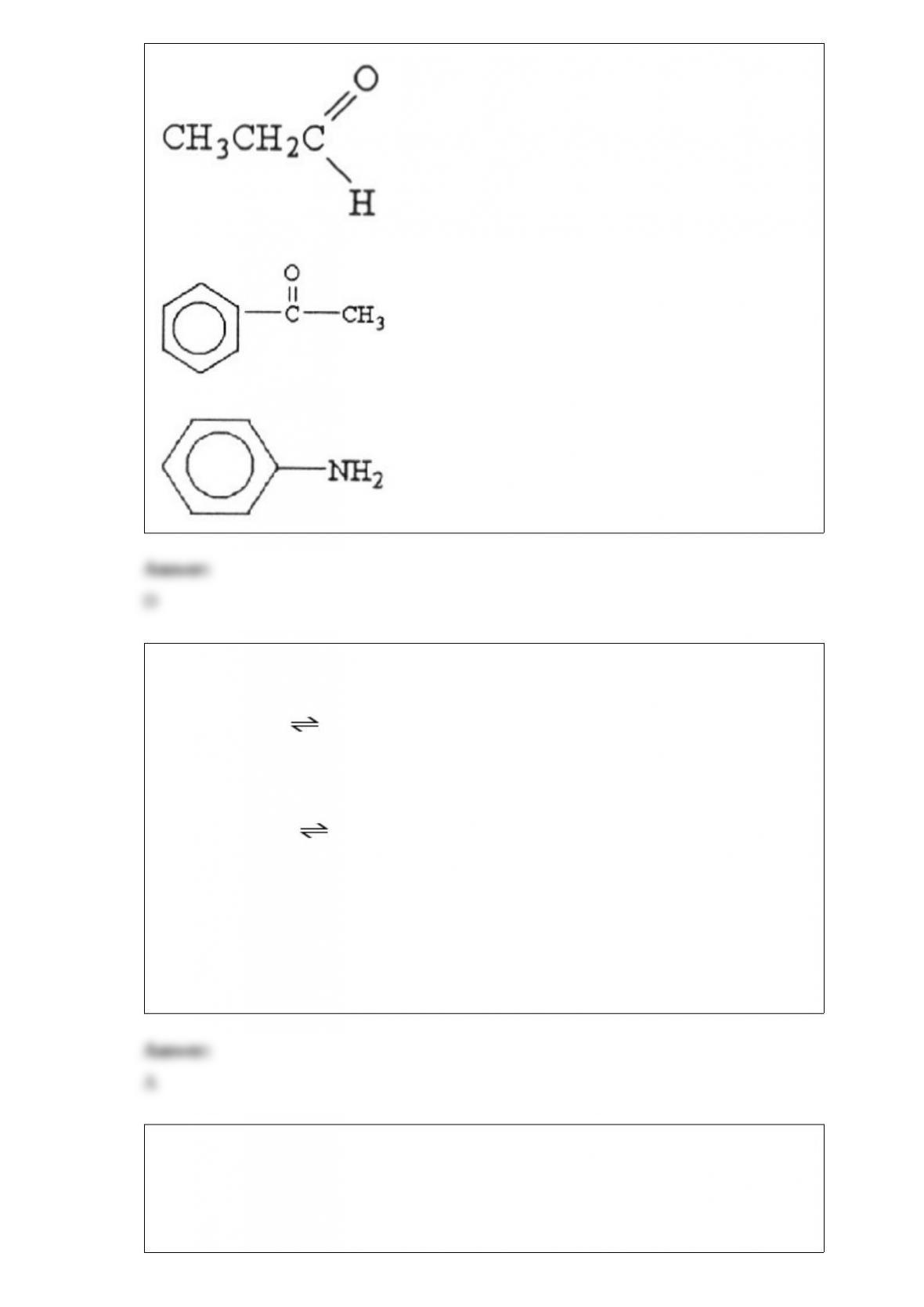
D) 32.3
E) -126
5) When the following equation is balanced, the coefficient of H2S is ________.
FeCl3 (aq) + H2S (g) → Fe2S3 (s) + HCl (aq)
A) 1
B) 2
C) 3
D) 5
E) 4
6) A meal containing a burger, fries, and a milkshake contains 53.0 grams of fat, 38.0
grams of protein, and 152 grams of carbohydrate. The respective fuel values for protein,
fat, and carbohydrate are 17, 38, and 17 kJ/g, respectively. If swimming typically burns
1100.0 kJ/hour, ________ minutes of swimming are required to completely burn off the
meal.
A) 4.78
B) 33.5
C) 62.5
D) 10.5
E) 286
7) Consider the following species when answering the following questions:
(i) PCl3(ii) CCl4(iii) TeCl4(iv) XeF4(v) SF6
In which of the molecules does the central atom utilize d orbitals to form hybrid
orbitals?
A) (i) and (ii)
B) (iii) only
C) (i) and (v)
D) (iii), (iv), and (v)
E) (v) only