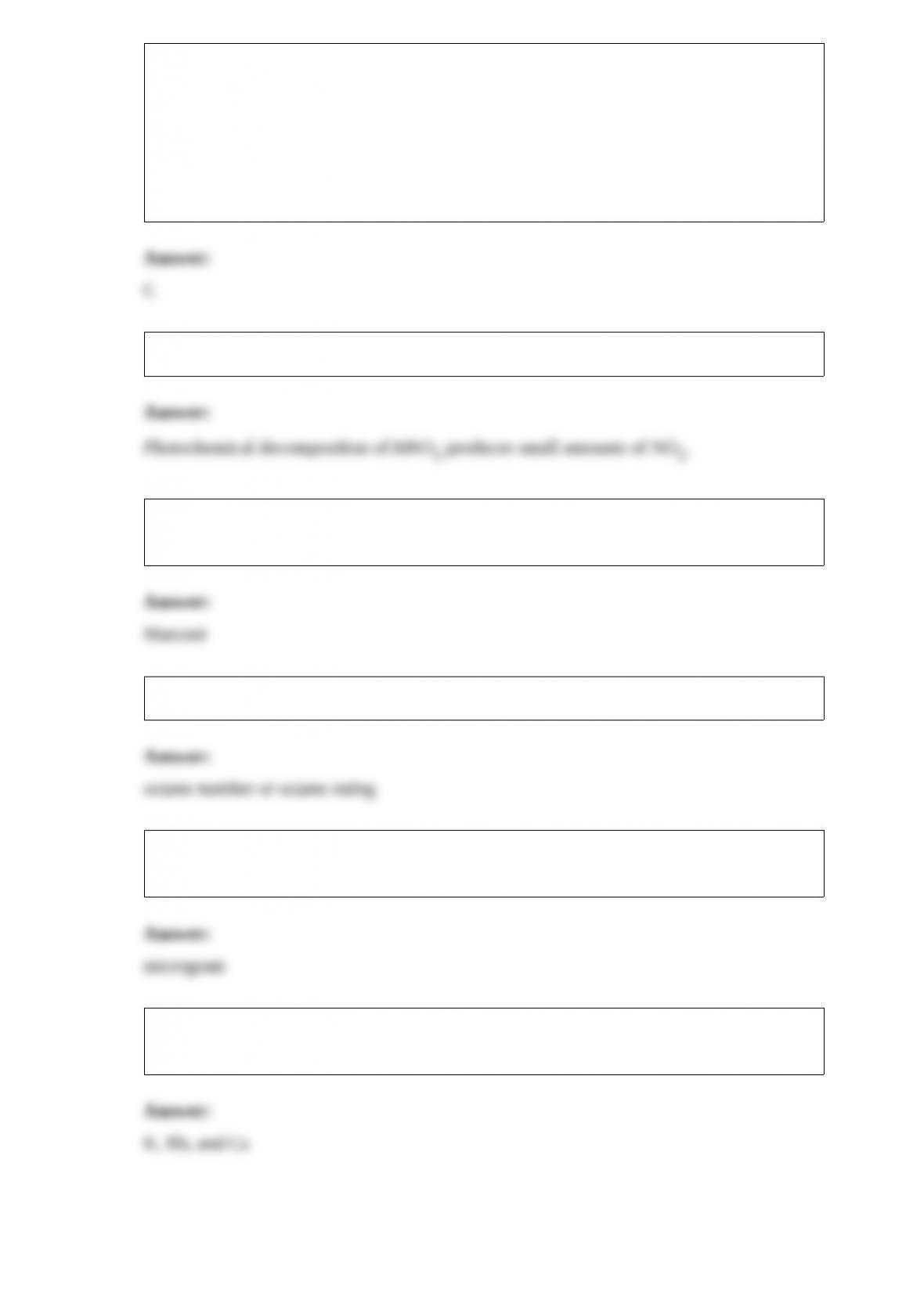
abundances of the isotopes are given in the table below. The average atomic mass of the
element is ________ amu.
A) 159.4
B) 162.0
C) 163.1
D) 161.5
E) 163.0
6) What change will be caused by addition of a small amount of HCl to a solution
containing fluoride ions and hydrogen fluoride?
A) The concentration of hydronium ions will increase significantly.
B) The concentration of fluoride ions will increase as will the concentration of
hydronium ions.
C) The concentration of hydrogen fluoride will decrease and the concentration of
fluoride ions will increase.
D) The concentration of fluoride ion will decrease and the concentration of hydrogen
fluoride will increase.
E) The fluoride ions will precipitate out of solution as its acid salt.
7) Which metal forms cations of differing charges?
A) K
B) Cs
C) Ba
D) Al
E) Sn
8) The kinetics of the reaction below were studied and it was determined that the
reaction rate increased by a factor of 9 when the concentration of B was tripled. The
reaction is ________ order in B.
A + B --> P