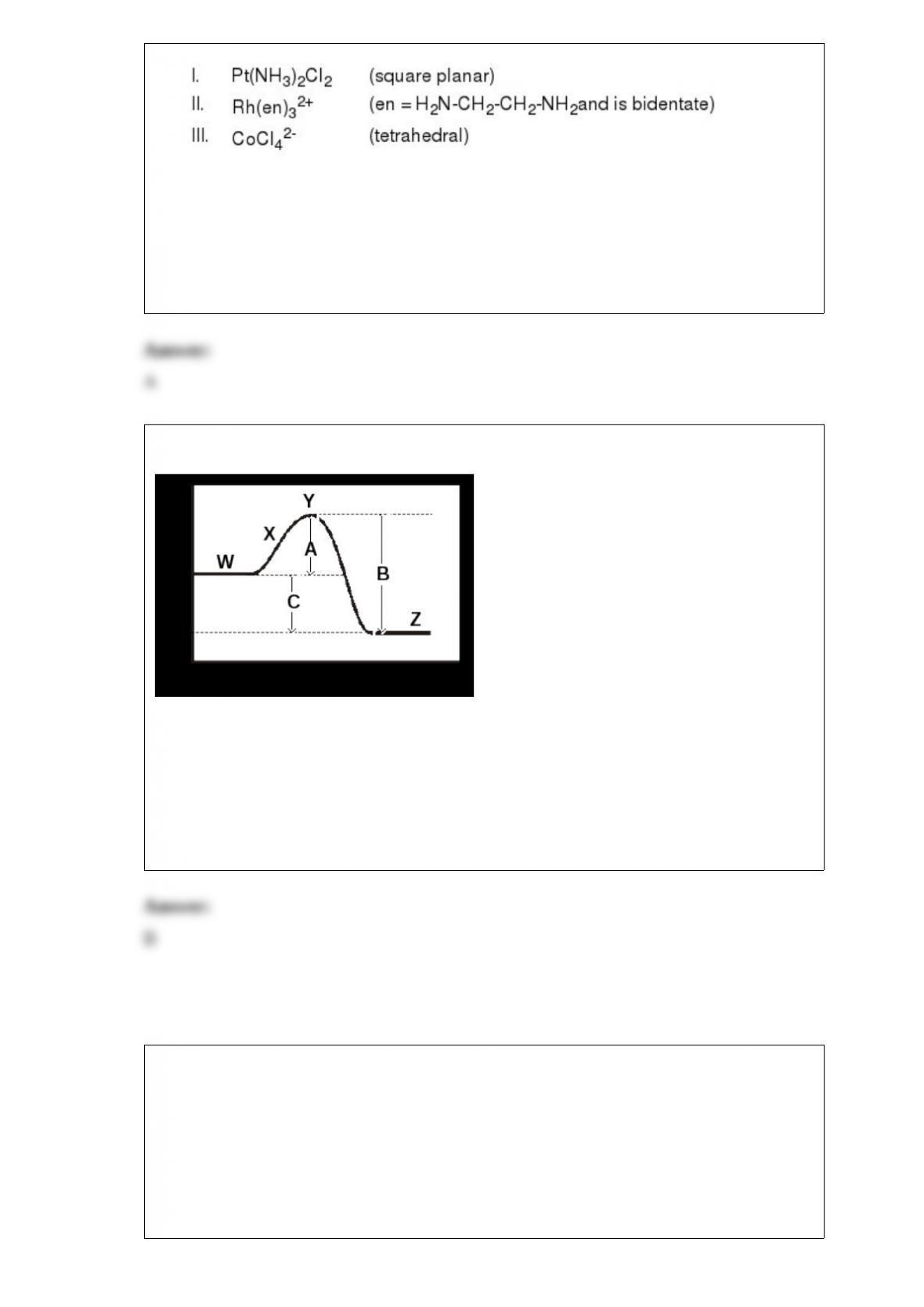
The questions below refer to the following system:
When cobalt(II) chloride is added to pure water, the Co2+ions hydrate. The hydrated
form then reacts with the Cl-ions to set up the equilibrium shown here.
Which statement below describes the change that the system will undergo if water is
added?
A) More chloride ions will be produced.
B) More water will be produced.
C) The equilibrium will shift to the right.
D) The color will become more blue.
E) There will be less of the hydrated cobalt ion at the new equilibrium position.
How many f orbitals have n=6?
A)2
B)7
C)10
D)5
E)18
What is reverse osmosis?
A)the application, to a concentrated solution, of a pressure that is greater than the
osmotic pressure, such that solvent flows from the concentrated solution to the dilute
solution
B)the application, to a dilute solution, of a pressure that is greater than the osmotic
pressure, such that solvent flows from the concentrated solution to the dilute solution
C)the application, to a concentrated solution, of a pressure that is greater than the
osmotic pressure, such that solute flows from the concentrated solution to the dilute
solution
D)the application, to a dilute solution, of a pressure that is greater than the osmotic
pressure, such that solute flows from the concentrated solution to the dilute solution
E)the application, to a concentrated solution, of a pressure that is greater than the
osmotic pressure, such that solvent flows from the dilute solution to the concentrated
solution