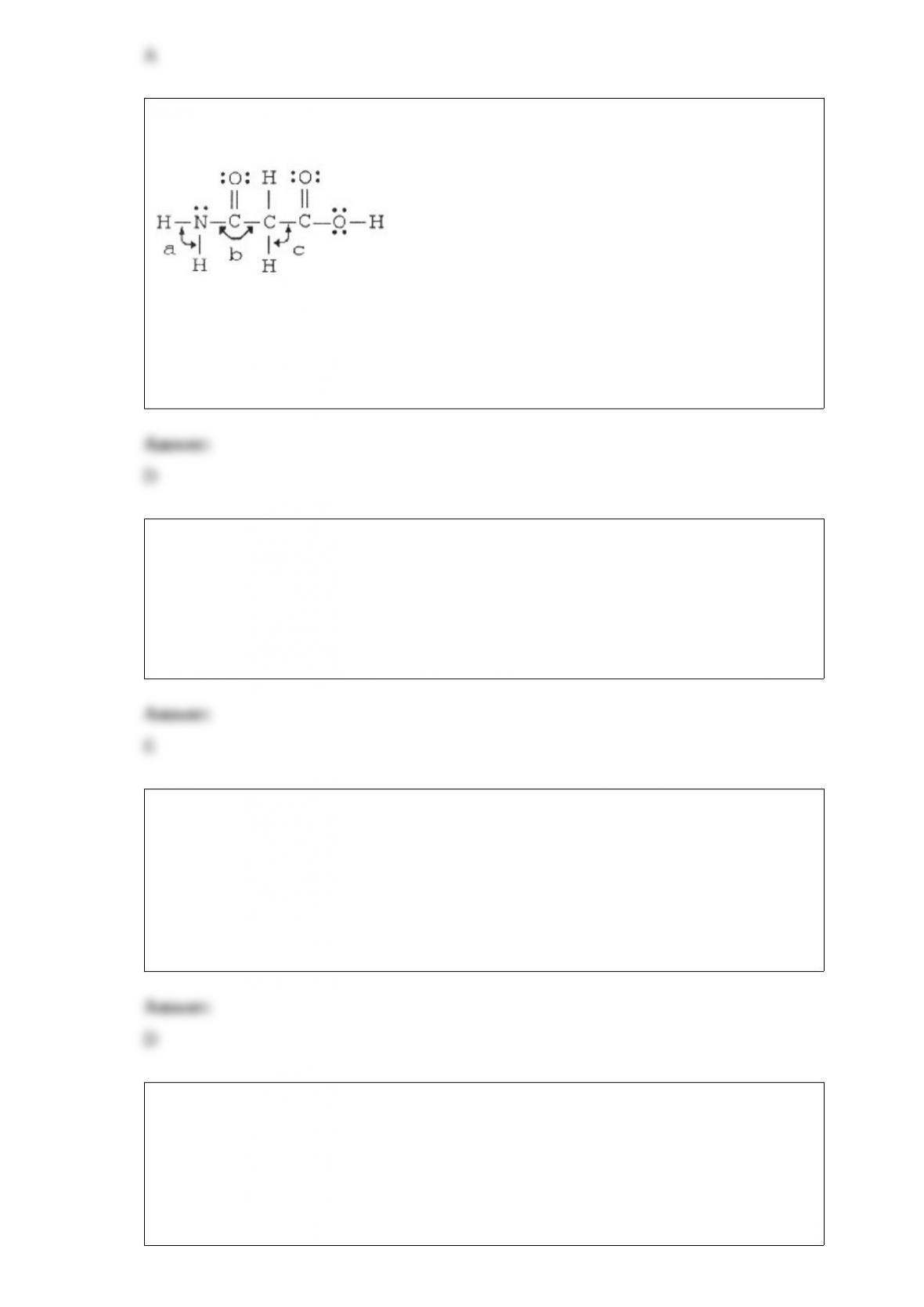
23) The bond angles marked a, b, and c in the molecule below are about ________,
________, and ________, respectively.
A) 90°, 90°, 90°
B) 120°, 120°, 90°
C) 120°, 120°, 109.5°
D) 109.5°, 120°, 109.5°
E) 109.5°, 90°, 120°
24) Formulas that show how atoms are attached in a molecule are called ________.
A) molecular formulas
B) ionic formulas
C) empirical formulas
D) diatomic formulas
E) structural formulas
25) In ionic bond formation, the lattice energy of ions ________ as the magnitude of the
ion charges _______ and the radii ________.
A) increases, decrease, increase
B) increases, increase, increase
C) decreases, increase, increase
D) increases, increase, decrease
E) increases, decrease, decrease
26) The Haber process is used to make ________ from ________.
A) HNO3, N2
B) O2, KClO3
C) NH3, N2
D) NO2, O2