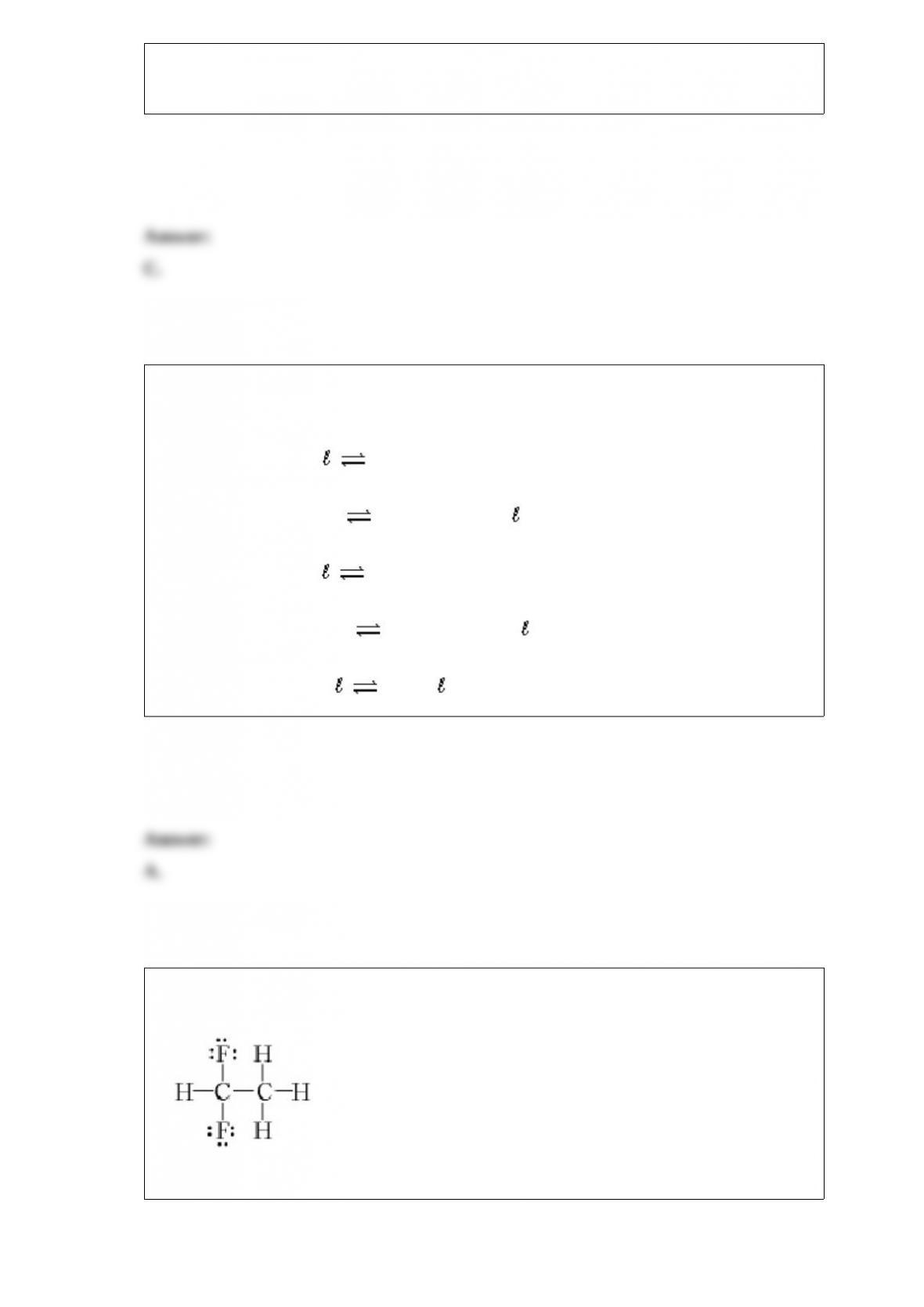
E.NH3
In positron emission tomography (PET), a positron emitted from an unstable isotope
travels a short distance before it is annihilated by
A.an electron, creating a proton that is detected by the instrument.
B.a neutron, creating two gamma rays that travel in opposite directions.
C.an electron, creating two gamma rays that travel in opposite directions.
D.an alpha particle, creating two protons that travel in opposite directions.
E.gamma ray, creating an electron that is detected by the instrument.
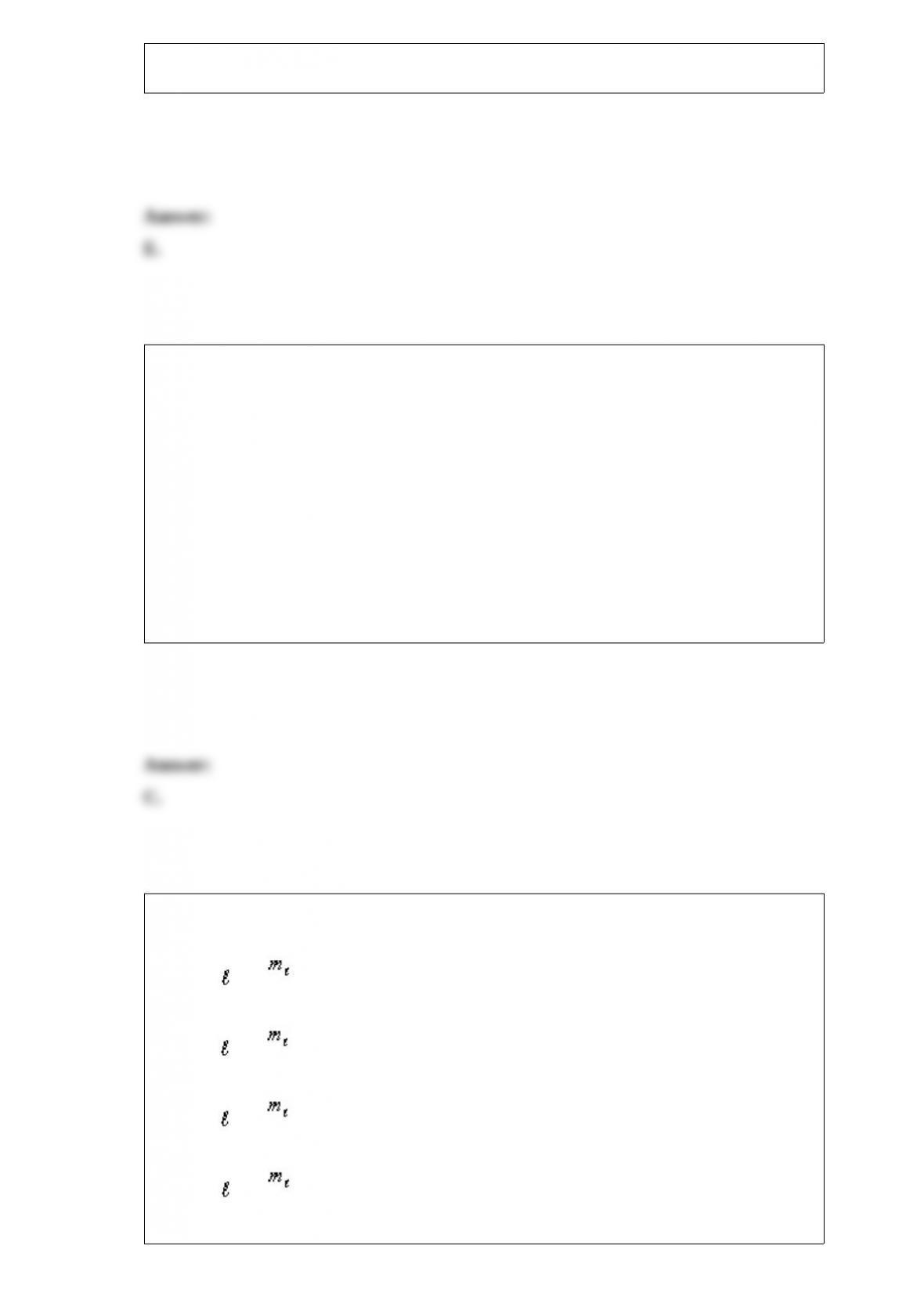
Which one of the following sets of quantum numbers is NOT allowed?
A.n = 7, = 0, = 0, ms = +1/2
B.n = 5, = 3, = "2, ms = +1/2
C.n = 4, = 2, = 0, ms = "1/2
D.n = 3, = 1, = "1, ms = +1/2