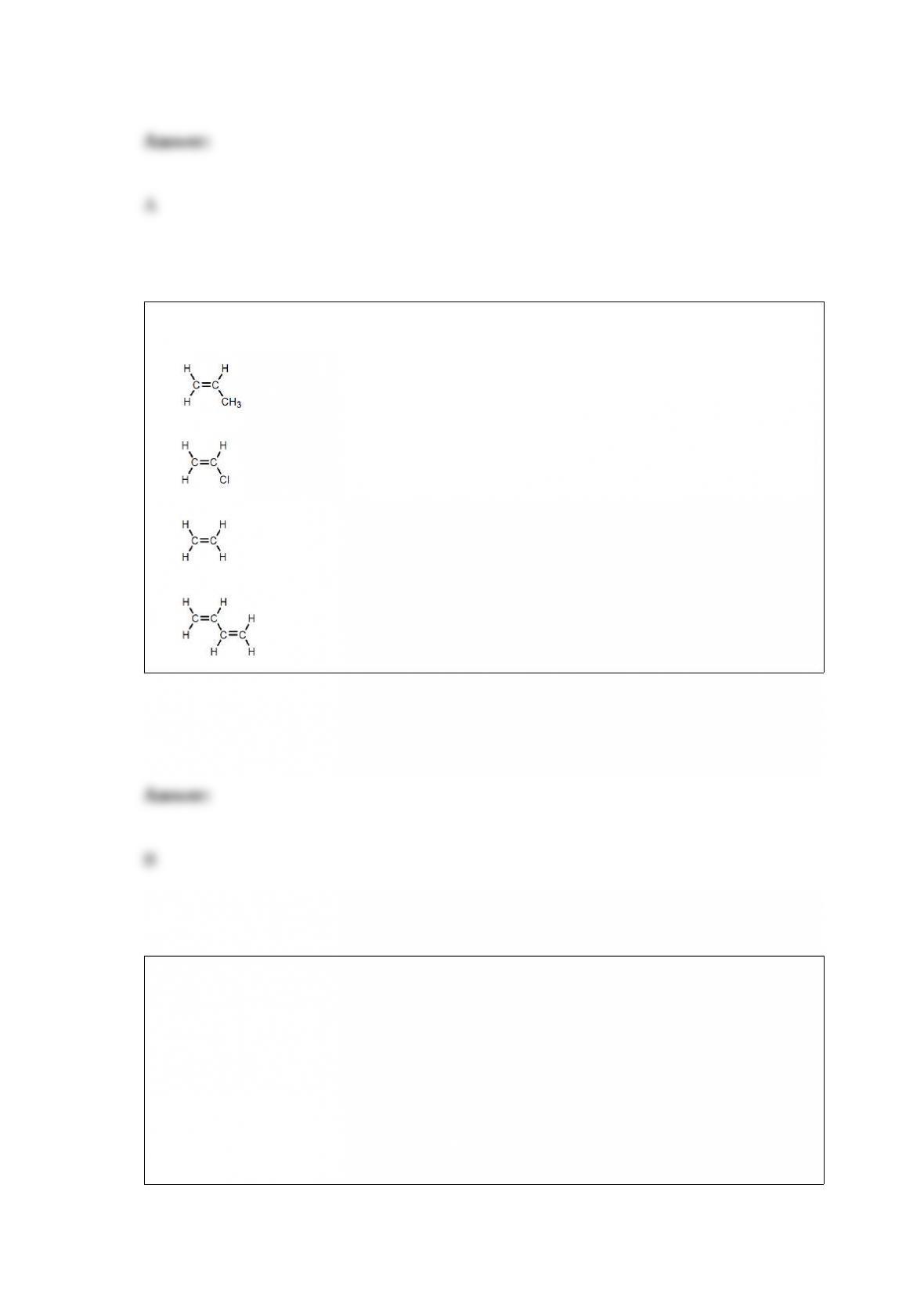
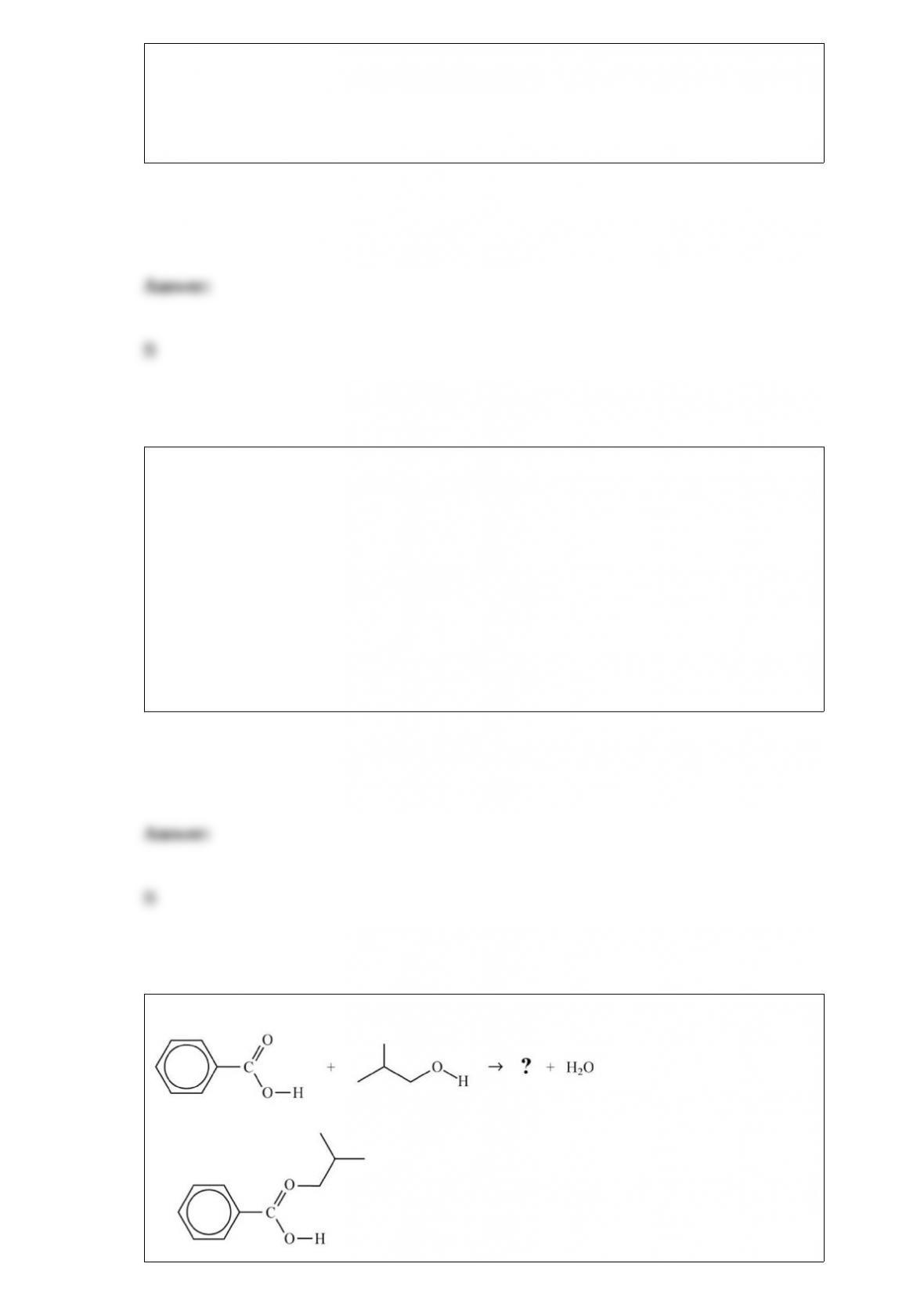
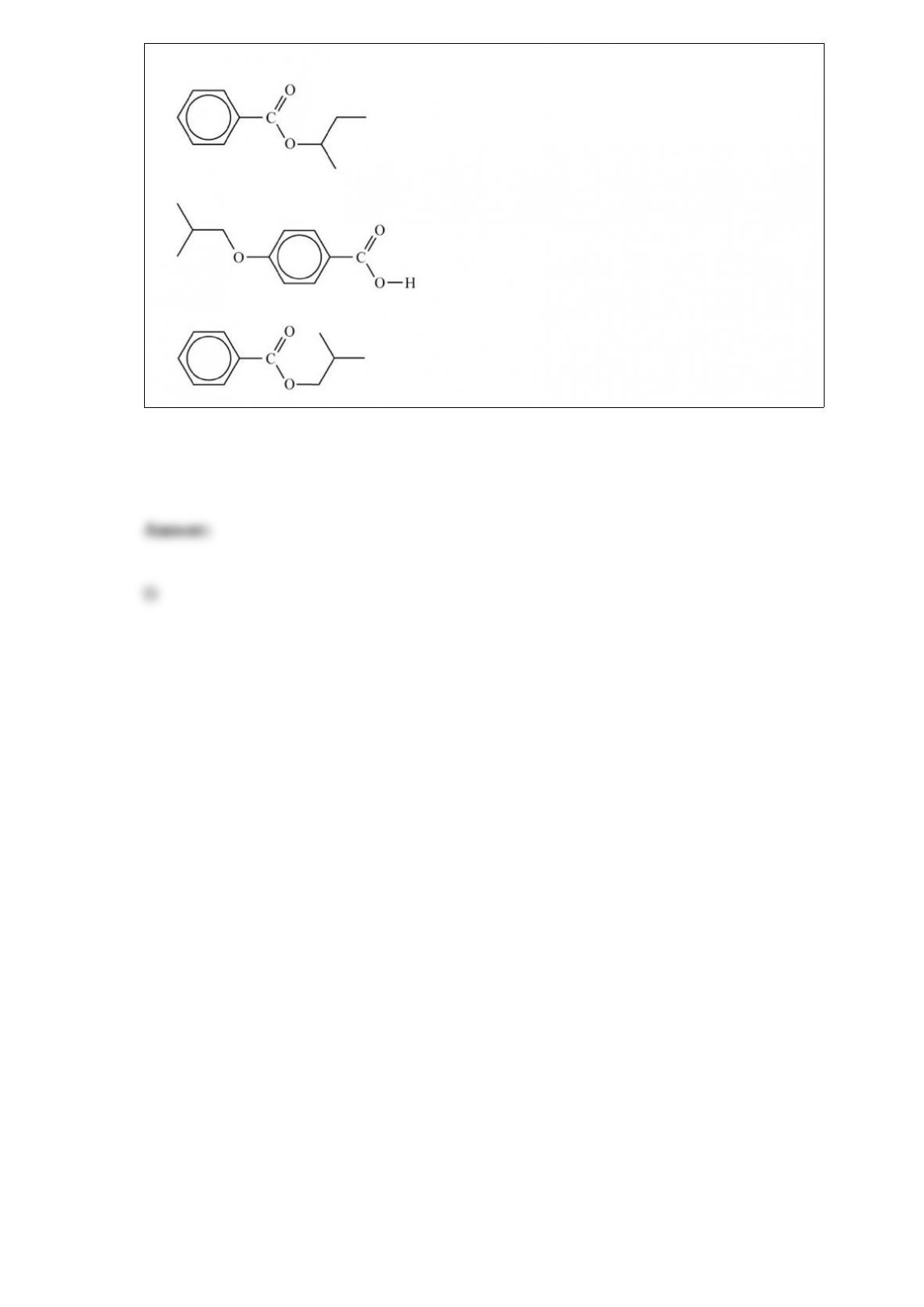
C. is the only isotope with equal numbers of protons, neutrons, and electrons.
D. is the only isotope of carbon that exists.
Calculate the percentage, by mass, of fluorine in sulfur hexafluoride (SF6), a potent
greenhouse gas.
A. 22.0%
B. 59.2%
C. 78.0%
D. 82.1%
The following warning appears on cans of diet beverages sweetened with aspartame:
"Phenylketonurics: Contains phenylalanine." Should you worry about drinking the
beverage?
A. No. For the majority of people, aspartame is a safe alternative to sugar.
B. Yes. The majority of people lack the enzyme needed to transform phenylalanine into
tyrosine.
C. No. Phenylalanine is an essential amino acid unavailable in any other food.
D. Yes. Phenylalanine causes mental retardation.