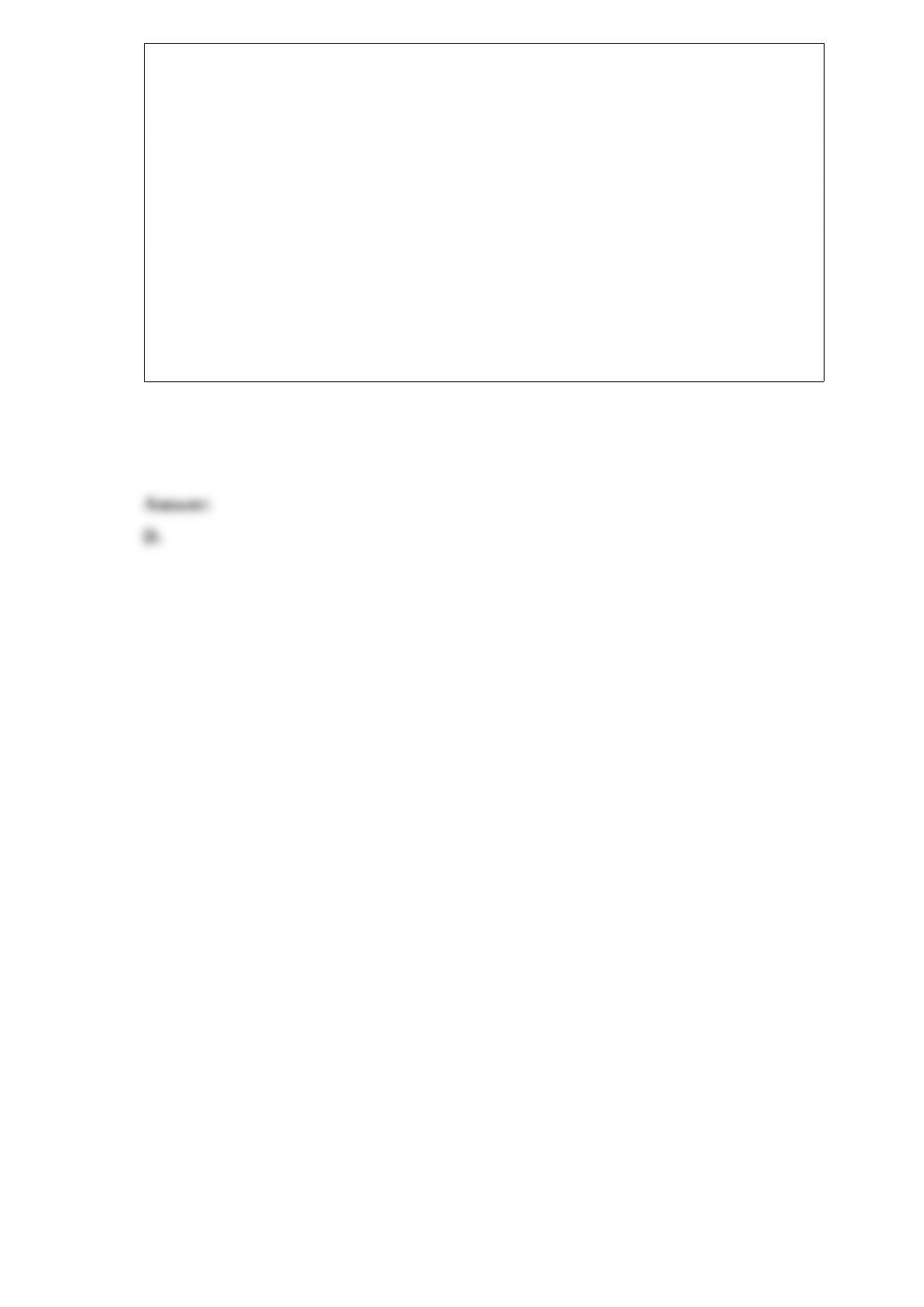
Consider the reaction A(aq) 2 B(aq) where Kc = 4.1 at 25 C. If 0.50 M A(aq) and 1.5
M B(aq) are initially present in a 1.0 L flask at 25 C, what change in concentrations (if
any) will occur in time?
A.[A] will decrease and [B] will decrease.
B.[A] will decrease and [B] will increase.
C.[A] will increase and [B] will decrease.
D.[A] will increase and [B] will increase.
E.[A] and [B] remain unchanged.
Write a balanced chemical equation which corresponds to the following equilibrium
constant expression.
A.PbF2(aq) Pb(s) + F2(aq)
B.PbF2(s) Pb2+(aq) + 2 F"(aq)
C.Pb2+(aq) + 2 F"(aq) PbF2(s)
D.Pb(s) + F2(aq) PbF2(aq)
E.PbF+(aq) + F"(aq) PbF2(aq)